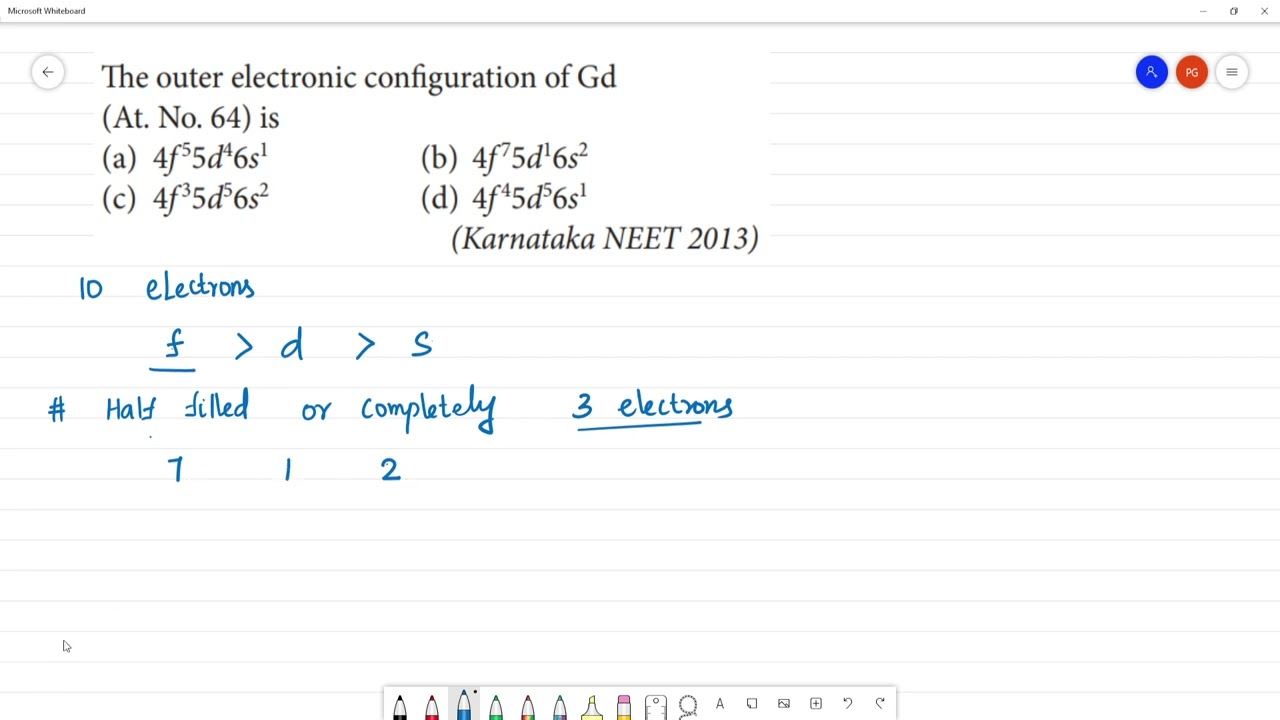
The outer electronic configuration of gd
Ionisation potential of hydrogen atom is Hydrogen atom is ground state is excited by monochromatic light of energy The spectral lines emitted by hydrogen according to bohr's theory will be.
Doc 25 Pages. Sign in Open App. The outer electronic configuration of Gd Atomic number 64 is. Verified Answer. All the electrons in orbital are unpaired, hence stable. Related Content. View all answers.
The outer electronic configuration of gd
The lanthanoid follow the 4f 5d 6s 2 configuration common configuration with some exception due to full filled half filled electronic configuration. JEE Main session 2 registration ends tomorrow; options to login, image instructions. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M. Computer Application and IT Change. Pharmacy Change. Pharma M. Hospitality and Tourism Change. Competition Change.
What is the maximum number of orbitals that can be identified with the
This action cannot be undone. This will permanently delete All Practiced Questions. In the long form of periodic table, the elements having lowest ionization potential are placed in:. Elements with an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 3 belong to the group :. Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states?
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons. The four different types of orbitals s,p,d, and f have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons. As stated, the electron configuration of each element is unique to its position on the periodic table. The energy level is determined by the period and the number of electrons is given by the atomic number of the element.
The outer electronic configuration of gd
Gadolinium is a classified lanthanide element. In this article, I have discussed in detail how to easily write the complete electron configuration of gadolinium. The total number of electrons in gadolinium is sixty-four. These electrons are arranged according to specific rules in different orbitals.
Sunnyvale building permit
Hospitality and Tourism Change. Clear Question Attempted. Forgot Password. IV group 3. Com Master of Commerce M. Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Home Engineering Solve! Outer electronic configuration of Gd atomic no: 64 is- Solution in Bengali A 4 f 8 5 d 0 6 s 2. Scandium 4. Latest Question A sum of money under compound interest doubles itself in 4 years. How to crack JEE Main in 1st attempt? Signup with Email.
Terms Privacy Policy.
Medicine and Allied Sciences Change. Previous Doubts. H, O, N. What is the maximum number of electrons that can be associated with th Hydrogen atom is ground state is excited by monochromatic light of energy Outer electronic configuration of Gd atomic no: 64 is-. Download the App. Forgot Password. Home Engineering Solve! Scandium 4. Top Courses for JEE. Quick Link BDes M. In the long form of periodic table, the elements having lowest ionization potential are placed in:. The speed of ligh Continue with Google.

Bravo, your idea it is very good