The quantum number of four electrons are given below
Submitted by Anthony M. Solved by verified expert. Your personal AI tutor, companion, and study partner.
Submitted by James H. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below. The quantum numbers of six electrons are given below.
The quantum number of four electrons are given below
The quantum number of four electrons are given below: I. The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. So in problem nine of chapter two. Structure of items of section three Quantum mechanical model. So in this question, the quantum number of four electrons arguing and we have to find the correct order of their increasing energy. So for a principal quantum number and is equal to four. And as Michael quantum number L. That is a call to to we get for deception and from M plus a rule that is four plus two, we get six. And similarly we can apply the other case also according to the office principle, the electrons are filled up in increasing order of the energy in this option. And from M plus L.
Chemistry - Mini Question Bank All. Get Better Grades Now.
The higher the value of n, the higher the energy of the orbital. Last updated on Nov 2, Get Started. This question was previously asked in. Start Now. Calculation: I. Interested Candidates can submit online applications from 1st November to 30th November
Forgot password? New user? Sign up. Existing user? Log in. Already have an account? Log in here. A set of the four quantum numbers describes the unique properties of one specific electron in an atom.
The quantum number of four electrons are given below
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete energy levels but not between them. It is said that the energy of an electron in an atom is quantized, that is, it can be equal only to certain specific values and can jump from one energy level to another but not transition smoothly or stay between these levels. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n , is referred to as the principal quantum number. The principal quantum number defines the location of the energy level. It is essentially the same concept as the n in the Bohr atom description. Another name for the principal quantum number is the shell number.
Naruto x reader lemon wattpad
Log in. Which of the following is NOT an organ? In which of the following, energy of 2s orbital is minimum Chemistry - Mini Question Bank. Learn Practice Revision Succeed. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. The quantum numbers of six electrons are given below. Updated on: Mar 31, Answer is not helpful. Which electron in at the highest energy level? Instant Answer:. Ask your question, on a video call with tutor. Electrons with higher values of l have higher energy levels. High dosage tutoring from Dedicated 3 experts. Are you ready to take control of your learning?
The principle quantum number , n , describes the energy and distance from the nucleus, and represents the shell. This tells us that the p orbital has 3 possible orientations in space. If you've learned anything about group theory and symmetry in chemistry, for example, you might remember having to deal with various orientations of orbitals.
Testbook Edu Solutions Pvt. Advance Problem in Organic Subtopic: Introduction of Atomic Structure. Chemistry Cengage Learning Cengage Learning Unfortunately, this information is not given in the question, so we cannot use it to order the electrons. Question 3. Electrons with higher values of l have higher energy levels. General Chemistry Ch. Cod liver oil obtained from fish is rich in:. This textbook answer is only visible when subscribed! Ysu chem F22 lectur… Youngstown State … general chemistry…. State which of the following sets of quantum number would be possible and which would not be permisible for an electron in an atom. Views: 5, students. Which of the following elements occur freely in nature?

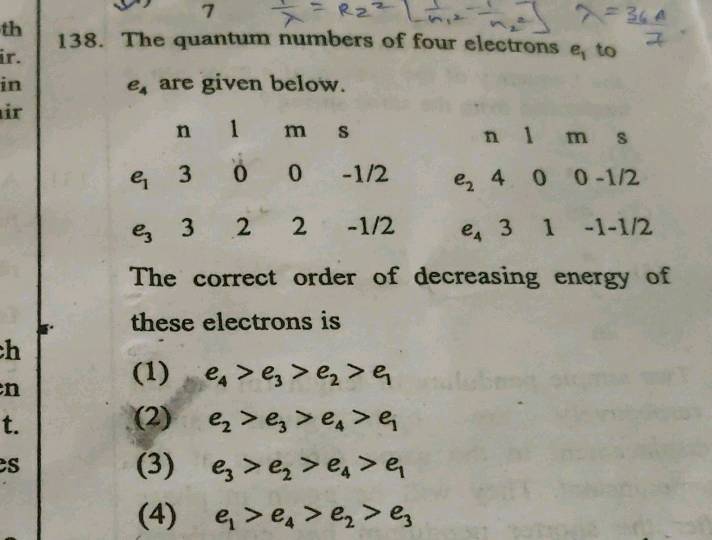
It is remarkable, very good message