The triple bond in ethyne is made up of
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond.
The triple bond in ethyne is made up of
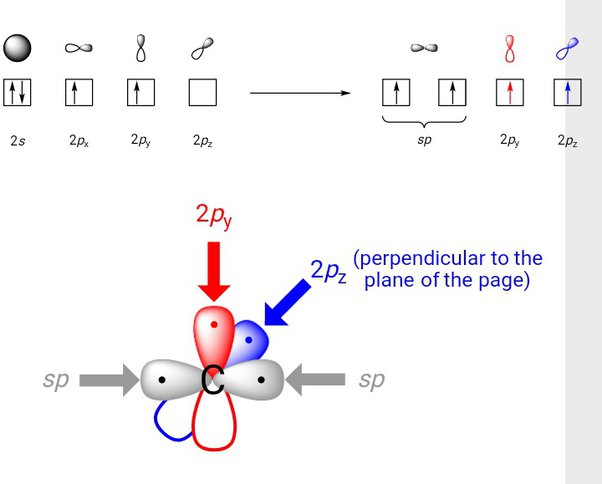
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes. When three pairs of electrons are shared between two carbon atoms, a triple bond is formed between the two carbon atoms. The distinguishing features of alkynes from the other hydrocarbons are the triple bond which exists between the carbon atoms. The triple-bonded carbon atoms of acetylene are sp hybridized. Sigma bonds between the atoms of carbon are obtained by a head-on overlapping of the two hybridized sp orbitals. The leftover orbitals of the carbon atoms overlap with each other along the internuclear axis in the 1s orbital of each hydrogen atom which results in the formation of one C-H sigma bond and 2 weaker pi bonds. These bonds are formed due s orbital overlapping. Each carbon atom uses only one of its three p-orbitals. Hence the two remaining p orbitals are occupied by a single electron. As it is the same with the other carbon atom, this permits the pairing of electrons which results in the formation of 2 pi bonds. The carbon-carbon triple bond is made stronger by the presence of one sigma bond.
Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. At last we will discuss some important questions related to zwitterion.
Structure of Triple Bond: What does triple bond mean? What is the structure of triple bond and what significance does it hold? We will try to answer to the above questions and others through this article. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond. Each carbon in ethene is attached to three other atoms as:.
The triple bond in ethyne is made up of
The chemical compound acetylene ethyne has the formula C 2 H 2. It is the simplest alkyne and a hydrocarbon. This colourless gas lower hydrocarbons are inherently gaseous is widely utilised as a fuel and chemical building material. It is usually treated as a solution because it is unstable in its pure state. Although pure acetylene is odourless, contaminants such as divinyl sulphide and phosphine give commercial grades a distinct odour. Acetylene is an unsaturated alkyne because its two carbon atoms are bonded together in a triple bond. He created potassium carbide K 2 C 2 by heating potassium carbonate with carbon at extremely high temperatures, interacting with water to release the new gas. Ethyne can be made from partially combustible methane.
Red roo basketball
In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Which is the weakest among the following types of bonds. Ethyne is more frequently known as acetylene. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall one sigma bond plus two pi bonds. Key Terms Make certain that you can define, and use in context, the key term below. Put your understanding of this concept to test by answering a few MCQs. Acetylene is a linear molecule with carbon-carbon distance of 1. Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the sigma bonds. These two sp hybrid orbitals are parallel and have a bond angle of degrees. D Two sigma and one pi bonds.
There are a number of interactive features in this resource:. Glossary: Any word with a glossary entry is highlighted like this.
Ethyne is used for preparing many organic solvents as well. The molecule which has the largest dipole moment amongst the following Grade The number of unpaired electrons in O 2 molecule is. The carbon-carbon bond order in benzene is A triple bond is formed between two carbon atoms when three pairs of electrons are shared between them. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall one sigma bond plus two pi bonds. The carbon-carbon bond in ethane structure A below results from the overlap of two sp 3 orbitals. Table of Contents. Uses of Alkynes The most common use of Ethyne is for making organic compounds such as ethanol, ethanoic acid, acrylic acid, etc.

Many thanks for the help in this question, now I will know.
So happens.