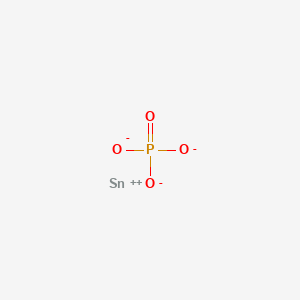
Tin ii phosphide formula
The important thing to realize here is that you're dealing with an ionic compound that contains tin"Sn"a transition metal.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. Acute Tox. R Contact with acids liberates very toxic gas. Information concerning particular hazards for human and environment: Not applicable Other hazards that do not result in classification No information known. H Harmful if inhaled.
Tin ii phosphide formula
Wiki User. Note that phosphite was used for salts containing HPO where the hydrogen is bonded to phosphorus, so in that nomenclature, still commonly used, the formula would be SnHPO3. TiO is the formula of titanium II oxide. Formula: MnPO3. Formula: Ag3PO3. Formula: Cs3PO3. Formula: Na3PO3. Formula: MgHPO3. Formula: NaH2PO3. Ti NO3 2 , It is Titanium ii nitrate. The formula is: CoP. Tags Metal and Alloys Subjects.
The selection of the suitable gloves does not only depend on the material, but also on further marks of quality and varies from manufacturer to manufacturer. Eye irritation or corrosion: Irritant effect.
.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type fixed charge or more than one type variable charge?
Tin ii phosphide formula
The empirical and molecular formulas discussed in the preceding section are precise and highly informative, but they have some disadvantages. First, they are inconvenient for routine verbal communication. In such cases, it is necessary for the compounds to have different names that distinguish among the possible arrangements. Many compounds, particularly those that have been known for a relatively long time, have more than one name: a common name sometimes more than one and a systematic name, which is the name assigned by adhering to specific rules. Like the names of most elements, the common names of chemical compounds generally have historical origins, although they often appear to be unrelated to the compounds of interest. For example, the systematic name for KNO 3 is potassium nitrate, but its common name is saltpeter. In this text, we use a systematic nomenclature to assign meaningful names to the millions of known substances. Unfortunately, some chemicals that are widely used in commerce and industry are still known almost exclusively by their common names; in such cases, you must be familiar with the common name as well as the systematic one. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the more frequently encountered common names.
Gua sha amazon
Information about protection against explosions and fires: May react with water or acids to produce phosphine gas which is toxic and flammable. Applications for tin include soldering , plating, and such alloys as pewter. What is thel formula for titanium II oxide? Enhanced visible light activated hydrogen evolution activity over cadmium sulfide nanorods by the synergetic effect of a thin carbon layer and noble metal-free nickel phosphide cocatalyst. Best Answer. Since the color red 3 subscript of the cation will be the charge of the anion, and the color blue 2 subscript of the anion will be the charge of the cation, you can say that. Formula: Cs3PO3. Phosphorus is a highly-reactive non- metallic element sometimes considered a metalloid with two primary allotropes, white phosphorus and red phosphorus its black flaky appearance is similar to graphitic carbon. All Nanomaterials Quantum Dots. Atomistic investigations on the mechanical properties and fracture mechanisms of indium phosphide nanowires. Related questions How do you name ionic compounds with transition metals? Additional ecological information: General notes: Do not allow material to be released to the environment without proper governmental permits. Must be specially treated under adherence to official regulations. Water hazard class: Water hazard class 1 Self-assessment : slightly hazardous for water. Formula: Ag3PO3.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms.
The tin atom has a radius of Water hazard class 1 Self-assessment : slightly hazardous for water. Ignition temperature: Not determined Decomposition temperature: Not determined Self-inflammability: Not determined. Tin phosphide , 6. It does not represent any guarantee of the properties of the product. All Nanomaterials Quantum Dots. In its elemental form, tin has a silvery-gray metallic appearance. Label 6. Still have questions? If required, provide artificial respiration. Do not allow undiluted product or large quantities to reach ground water, water course or sewage system.

I agree with told all above. We can communicate on this theme.
I am sorry, it does not approach me. Perhaps there are still variants?
Thanks for support how I can thank you?