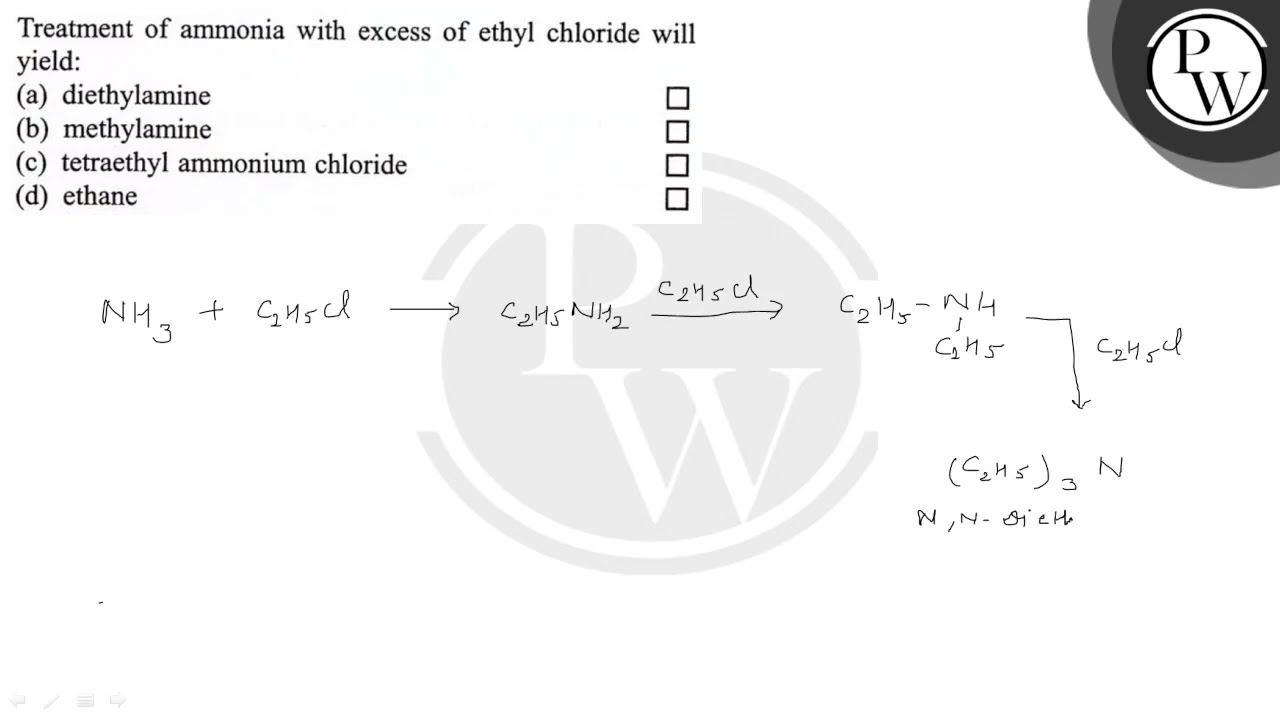
Treatment of ammonia with excess ethyl chloride
Diethyl amine.
Hence, treatment of ammonia with excess ethyl chloride gives Tetraethyl ammonium chloride. Mistake Points. Additional Information l. Last updated on Mar 31, In this year's recruitment cycle, a total of vacancies were released.
Treatment of ammonia with excess ethyl chloride
Hence, treatment of ammonia with excess ethyl chloride gives Tetraethyl ammonium chloride. Mistake Points. Additional Information l. Last updated on Mar 31, In this year's recruitment cycle, a total of vacancies were released. This is a golden opportunity for those candidates who want to get into the teaching profession in the state of Uttar Pradesh. For the upcoming cycle, a total of vacancies are expected. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
JEE Advanced. The homolytic fission of a covalent bond liberates :.
.
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. Rimsha Ali ; Shivaraj Nagalli. Authors Rimsha Ali 1 ; Shivaraj Nagalli 2. Hyperammonemia is a metabolic condition characterized by raised levels of ammonia, a nitrogen-containing compound. Ammonia is a potent neurotoxin. Hyperammonemia most commonly presents with neurological signs and symptoms that may be acute or chronic, depending on the underlying abnormality. Hyperammonemia should be recognized early and treated immediately to prevent the development of life-threatening complications such as cerebral edema and brain herniation.
Treatment of ammonia with excess ethyl chloride
Ethanoyl chloride is taken as a typical acyl chloride. Any other acyl chloride will behave in the same way. Simply replace the CH 3 group in what follows by anything else you want. Ethanoyl chloride reacts violently with a cold concentrated solution of ammonia. A white solid product is formed which is a mixture of ethanamide an amide and ammonium chloride. Notice that, unlike the reactions between ethanoyl chloride and water or ethanol, hydrogen chloride isn't produced - at least, not in any quantity. Any hydrogen chloride formed would immediately react with excess ammonia to give ammonium chloride. The first stage the addition stage of the reaction involves a nucleophilic attack on the fairly positive carbon atom by the lone pair on the nitrogen atom in the ammonia.
Reactive outdoor tent
Bihar Vidhan Sabha Security Guard. UP Police Constable. Start Now. SSC Scientific Assistant. Cochin Shipyard Draftsman Trainee. Allahabad High Court Computer Assistant. Haryana Police Commando. BIS Stenographer. EPFO Stenographer. The chief ore of aluminium is. Maharashtra Zilla Parishad Supervisor. Chandigarh TGT. Diethyl amine. NFL MT. JSSC Clerk.
Hence, treatment of ammonia with excess ethyl chloride gives Tetraethyl ammonium chloride. Mistake Points. Additional Information l.
RBI Office Attendant. WRD Maharashtra. Rajasthan Police Constable. Haryana PGT. IOCL Apprentice. GATE Mathematics. Maharashtra Nagar Parishad Engineering Services. Allahabad High Court Computer Assistant. Rajasthan Home Guard. Vizag Steel Junior Trainee. Army Havildar SAC. Osteoclasts are associated with which of the following? Navy Tradesman Mate. Maharashtra Planning Assistant.

The authoritative answer, cognitively...