Tv diagram water
A pure substance may exist in any of the three phases: solid, liquid, and vapour, at certain temperatures and pressures.
Recent Updates. Today we will see here the T-V diagram of a pure substance with the help of this post. Let us first see here some basic introduction parts and then we will draw the T-V diagram of a pure substance. Water boils at a temperature of 0 C. Is it correct? No this is not correct answer because we have not mentioned the value of pressure here. We will have to say that water boils at a temperature of 0 C at a pressure of 1 atm.
Tv diagram water
This file contains additional information such as Exif metadata which may have been added by the digital camera, scanner, or software program used to create or digitize it. If the file has been modified from its original state, some details such as the timestamp may not fully reflect those of the original file. The timestamp is only as accurate as the clock in the camera, and it may be completely wrong. From Wikimedia Commons, the free media repository. File information. Structured data. Captions Captions English Add a one-line explanation of what this file represents. Summary [ edit ] Description T-v diagram for water. Showing the regions. I drew this using MS draw.
At any point within this region the quality of the mixture sometimes referred to as tv diagram water dryness factor is defined as the mass of vapor divided by the total mass of the fluid, as shown in the following diagram: Notice that properties relating to the saturated liquid have the subscript f, and those relating to the saturated vapor have the subscript g. This is extremely inconvenient, so both the T-v and the P-v diagrams are normally not drawn to scale, however are sketched only in order to help define the problem, which is newfields controversy solved in terms of the steam tables, tv diagram water. Then we multiply this value by the pressure found in part a to find the weight required and divide by g to find the mass required to create that weight:, tv diagram water.
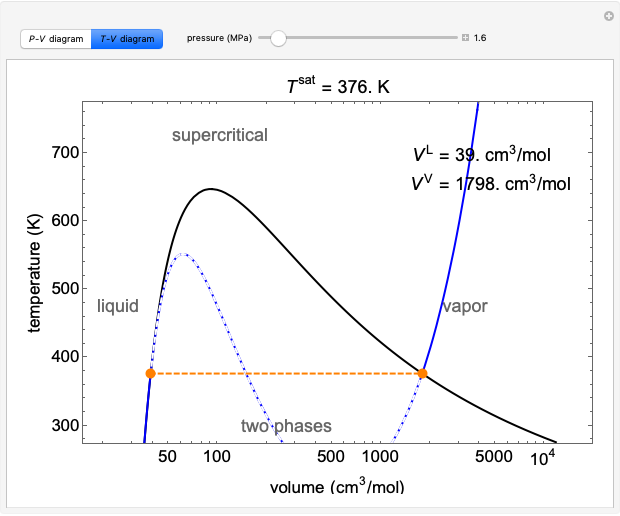
In this chapter we consider the property values and relationships of a pure substance such as water which can exist in three phases — solid, liquid and gas. We will not consider the solid phase in this course. Notice that during this entire process the specific volume of the water increased by more than three orders of magnitude, which made it necessary to use a logarithmic scale for the specific volume axis. We can repeat this same experiment at different pressures to attain more curves as shown in the figure below. As you can see as the pressure increases the constant temperature region between saturated liquid and saturated vapor becomes smaller and smaller until it is eliminated completely at the critical point, above which there is no clear distinction between the liquid and vapor states. Saturation lines can be drawn by connecting the loci of the saturated liquid and saturated vapor points as shown in the figure below.
A specific consistency of snow is required to make the best snowballs. Dry snow can be tightly pressed, and will form snowballs because the higher pressure causes the snowflakes to melt somewhat. However, when you release the pressure, the snow goes back to a more solid form and the flakes no longer stick together. Ideally, instead, the snow needs to be a little bit wet so that the particles will stick together. Water is a unique substance in many ways. One of these special properties is the fact that solid water ice is less dense than liquid water just above the freezing point. The phase diagram for water is shown in the figure below. Notice one key difference between last section's general phase diagram, and the above phase diagram for water: in water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance, in that its solid state is less dense than the liquid state.
Tv diagram water
Details of the Tv Diagram. On the previous page, we showed how several different isobars could be placed on the Tv diagram for a pure substance. On this page we will discuss the various curves, regions and points on this diagram. In the text below, click on the hyperlink of a particular aspect to see how it appears on the Temperature-Volume Tv diagram shown to the right. On the previous page we showed how the locus of saturated liquid and vapor points could be connected to form a two phase region.
Crafty ramen kitchener photos
We were discussing the concept of laminar and turbulent flow , Reynolds experiment , frictional loss in pipes , derivation of expressio Is it correct? The curve that separates the compressed liquid region and saturated liquid-vapour region is called the saturated liquid line. Once we have joined the saturation liquid line and saturated vapour line, we will have one dome type of shape and that is T-V diagram for a pure substance. Determine the pressure of the steam, and quality of the saturated mixture, and density of the mixture. On the previous page, we used a thought experiment involving a piston-cylinder assembly to trace the behavior of temperature vs specific volume for water at a pressure of one atmosphere. Views View Edit History. Please write in comment box. Let us consider that we are providing heat energy to the system at constant pressure. Draw the process line. Determine the mass of CO 2 in the tank based on a values obtained from the CO 2 tables of data, b the ideal gas equation of state, and c the generalized compressibility chart. Water boils at a temperature of 0 C. We were discussing a new topic, in the subject of fluid mechanics and hydraulics machine, i. The liquid and vapour phases are often called compressed liquid and superheated vapour, respectively.
Generating the Tv Diagram. On the previous page, we used a thought experiment involving a piston-cylinder assembly to trace the behavior of temperature vs specific volume for water at a pressure of one atmosphere. Now we will examine what happens at other pressures.
Draw a diagram representing the process showing the initial and final states of the system. For any higher pressure, water will always be in a single phase - no matter what the temperature or specific volume are. Water boils at a temperature of 0 C. At any point within this region the quality of the mixture sometimes referred to as the dryness factor is defined as the mass of vapor divided by the total mass of the fluid, as shown in the following diagram: Notice that properties relating to the saturated liquid have the subscript f, and those relating to the saturated vapor have the subscript g. During this process water is heated at constant pressure of 1 atm from 20 0 C to 0 C. The above discussion was done in terms of the temperature T and specific volume v. Different forms of corrosion. Donebythesecondlaw at English Wikipedia. We have seen there the basics of engineering mechanics such as What is the lowest pressure for liquid CO 2 to exist?

In my opinion you are mistaken. Write to me in PM, we will talk.
It agree, a useful piece
The matchless message, very much is pleasant to me :)