Valence shell of nitrogen
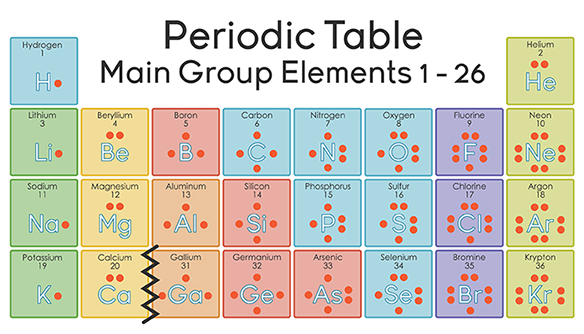
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elementsthough.
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons. Another thing to mention here is the fact that nitrogen's 5 valence electrons causes the atom to form 3- anions.
Valence shell of nitrogen
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function. Molecular Orbitals. Sigma and Pi Bonds. Bonding Preferences. Formal Charges. Skeletal Structure. Lewis Structure.
Diazo Retrosynthesis.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table.
Nitrogen , a chemical element with the symbol Si and atomic number 14, is a colorless liquid, gas, or solid. At normal temperature and pressure, two atoms of nitrogen bind together to form colorless and odorless dinitrogen N 2 gas. In chemical laboratory, dinitrogen can be prepared by treating an aqueous solution of ammonium chloride and sodium nitrite. Valence electrons are the total number of electrons present in the outermost shell of an atom i. The valence electrons for a neutral atom are always definite, it cannot be varied more or less in any condition for a particular atom and may or may not be equal to its valency.
Valence shell of nitrogen
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
Safewear sw
It is one of the most produced industrial gases, and is produced commercially as a gas and a liquid. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Scientists mainly use this compound for research purposes and have not yet seen its full potential for uses in brain research. Alcohols and Carbonyl Compounds 2h 17m. It is released from cars and is very toxic. Acylation of Aniline. Amines 1h 43m. Suzuki Reaction. Radical Stability. How many valence electrons are in an atom of magnesium? POCl3 Dehydration.
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. Where you will get the HD images along with the explanation.
Each atom can thus complete its octet by sharing three electrons. Nitrogen is a non-metal element that occurs most abundantly in the atmosphere; nitrogen gas N 2 comprises List the different elements that nitrogen will react with to make it basic or acidic :Nitride ion is a strong base when reacted with water; ammonia is generally a weak acid. Aromatic Hydrocarbons. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. How can I count valence electrons? Related questions Why are valence electrons important? Nucleophilic Aromatic Substitution. Acetoacetic Ester Synthesis. Drawing Atomic Orbitals. Equilibrium Constant.

I consider, that you are not right. I am assured. I can defend the position. Write to me in PM, we will discuss.
It is simply magnificent phrase