What are isotopes and isobars give examples
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers.
The atoms of an element with the same atomic number but different atomic masses are termed isotopes. On the other hand, the elements with the same atomic mass but different atomic numbers are called Isobars. The chemical reactivity of isotopes is not affected as the number of electrons remains the same. But Isobars contain different numbers of electrons or protons which affect their reactivity. This Chemistry article focuses on the meaning, differences, examples, and uses of Isotopes and Isobars. An isotope of a chemical element is one of two or more species of atoms that share the same atomic number and position in the periodic table.
What are isotopes and isobars give examples
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. An example of two isotopes and isobars is nickel and iron. These both have the same mass number, which is 58, whereas the atomic number of nickel is 28, and the atomic number of iron is Let us consider an example of 2 things, which appear to be the same in colour and in their physical appearance, such that we cannot distinguish between them. However, when we measure the weight of these two, then we find a difference. We can relate the isotopes concept with this example. As we all know, every atom is made of electrons, protons, and neutrons. The nucleus is made of only protons and neutrons, and the electrons revolve around the nucleus.
As a result, the isotopes of an element have the same atomic number but different atomic masses. Please Login to comment
Isobars are a group of elements that have the same mass number but different atomic numbers. In an isobar, we have different numbers of protons but the same number of nucleons, i. An example of isobar is carbon and nitrogen as they both have 14 nucleons in their nucleus but different atomic numbers, the atomic number of carbon is 6 and the atomic number of nitrogen is 7. The isobar has somewhat the same physical properties but different chemical properties. In this article, we will learn about isobars, their examples, their differences with isotopes and others in detail. Isobars are a group of elements from the periodic table that have different atomic numbers but their mass number are the same.
The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements. Protons with unit positive charge and mass of 1. The nucleus of an atom can be represented as:. Atoms of the same element having the same atomic number but different mass numbers are called isotopes. It arises due to the difference in the number of neutrons in the nucleus. The chemical properties of isotopes of the elements are the same, however, their physical properties are different. Isotopes are of two types; stable and radioactive unstable. Stable isotopes occur in free states without disintegration. Atoms of different elements having different atomic numbers but the same mass numbers are called isobars. Isobar possesses different chemical properties, but the same physical properties.
What are isotopes and isobars give examples
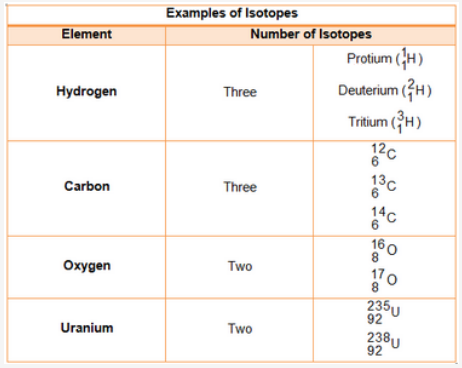
Isotopes are forms of an element that have different numbers of neutrons. All isotopes of an element have the same atomic number and number of protons , but they have different atomic masses from each other. Isotopes of an element share similar chemical properties, but have different nuclear properties. Every element has isotopes. The 81 stable elements have isotopes. But, elements with stable isotopes also have radioactive isotopes or radioisotopes. The radioactive elements , on the other hand, have no stable isotopes. Over radioactive isotopes have been identified. Some of the radioactive isotopes are natural, while others have only been produced in the laboratory.
Lsu florida baseball box score
Isobars in chemistry are defined as groups of elements that have different atomic numbers but similar mass numbers. All these have the same number of protons, but they differ in the number of neutrons. Skip to content. However, when we measure the weight of these two, then we find a difference. Please go through our recently updated Improvement Guidelines before submitting any improvements. Download Now. The physical properties are often similar. The table added below shows the following condition,. Contribute your expertise and make a difference in the GeeksforGeeks portal. Here, both carbon and nitrogen have the same mass number but different atomic numbers. Acetic Acid Formula. There are various examples in the periodic table that are isobars, i. Isobars are atoms of different elements. Isobar is an element that differs in chemical properties, but it has similar physical properties.
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers.
Isotopes can be identified by the same number of protons and a different number of neutrons. Let us look at the difference between isotopes and isobars, as tabulated below. Share your suggestions to enhance the article. Skip to content. They have different atomic numbers. Examples Of Condensation. Isobars are a group of elements with the same mass numbers but different atomic numbers. This happens because they have different atomic numbers but the sum of protons and neutrons in their nucleus is different. Improved By :. Moreover, the atomic number is equal to the number of protons. The atoms of an element with the same atomic number but different atomic masses are termed isotopes.

0 thoughts on “What are isotopes and isobars give examples”