What is the correct name for n2o4
The Dinitrogen tetroxide molecule contains a total of 6 atom s.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons.
What is the correct name for n2o4
.
Read Edit View history.
.
Explore more content. Cite Download 4. Since as early as the s, dinitrogen tetroxide N2O4 has been regarded as a promising oxidizer in rocket propulsion systems. In more recent times, its predecessor, mixed oxides of nitrogen MON , remains a top contender among oxidizers, due to its unique characteristics such as low freezing temperature and compatibility with common spacecraft materials. Today, these N2O4-based oxidizers are the preferred choice in many upper stages, launch escape systems, reaction control systems, liquid apogee engines, and in-space primary propulsion systems. N2O4-based oxidizers are a key factor in rocket propulsion, and thoroughly understanding their history, development, characteristics, synthesis, and composition analysis are crucial for space exploration today and into the future. To fully understand and predict the physical properties of a MON sample, it is important to measure and quantify its chemical composition.
What is the correct name for n2o4
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Markiplier try not to laugh 4
The gas is essentially pure nitrogen dioxide, which is condensed into dinitrogen tetroxide in a brine-cooled liquefier. Confirm Valid email address confirmed. The Dinitrogen tetroxide compound may have different names depending on the various different situations of industrial applications. BBC News in Spanish. Such dissociative gas Brayton cycles have the potential to considerably increase efficiencies of power conversion equipment. Descriptive inorganic chemistry 6th ed. Home Formula This Compound. The chemical formula of Dinitrogen tetroxide shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type. Freeman, p. The law of conservation of mass dictates that the quantity of each element given in the chemical formula does not change in a chemical reaction. Refractive index n D. The anhydrous nitrates concerned are themselves covalent, and many, e. The Dinitrogen tetroxide molecule contains a total of 6 atom s. PubChem CID. Dinitrogen tetroxide can also be produced by heating metal nitrates.
As with ionic compounds, the system that chemists have devised for naming covalent compounds enables us to write the molecular formula from the name and vice versa. In this and the following section, we describe the rules for naming simple covalent compounds. We begin with inorganic compounds and then turn to simple organic compounds that contain only carbon and hydrogen.
It is also the primary oxidizer for Russia's Proton rocket. Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen pentoxide. Various anhydrous transition metal nitrate complexes can be prepared from N 2 O 4 and base metal. Dinitrogen tetroxide can also be produced by heating metal nitrates. Pedro Paulet, sabio multidisciplinario in Spanish. It is then much easier to compress to start the entire cycle again. Space-filling model. In the first step, the ammonia is oxidized into nitric oxide :. Thus, each side of the chemical equation must represent the same quantity of any particular element based on the chemical formula. Precautionary statements. LCCN Archived PDF from the original on 7 May

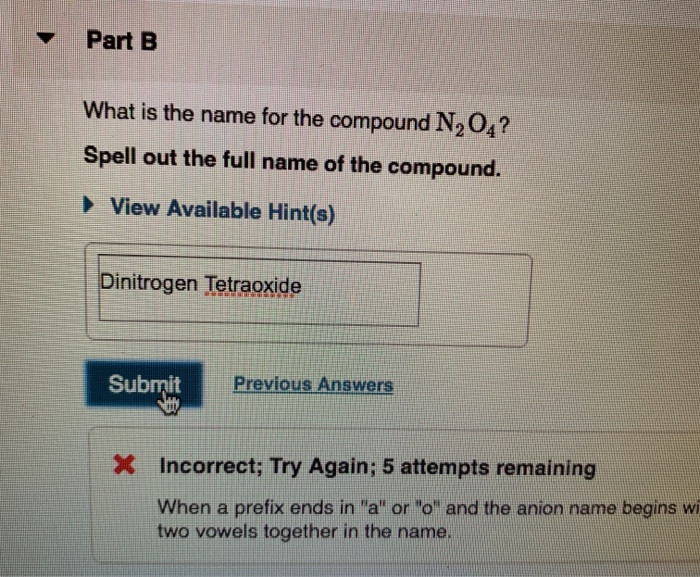
Remember it once and for all!