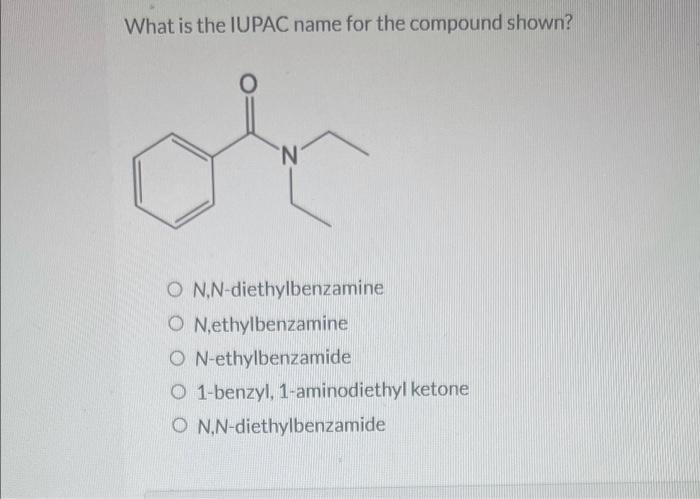
What is the iupac name for the compound shown
In order to give compounds a name, certain rules must be followed. This is to give consistency to the names. It also enables every compound to have a unique name, which is not possible with the common names used for example in industry. We will first look at some of the steps that need to be followed when naming a compound, and then try to apply these rules to some specific examples.
In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type s of functional group s present on or within the parent chain. Other groups which are attached to the parent chain are called substituents.
What is the iupac name for the compound shown
.
Give the structural representation of each of the following organic compounds:. There is an ethyl group on the second carbon atom. There are three carbon atoms in the longest chain, therefore the prefix is propan.
.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular?
What is the iupac name for the compound shown
One way of checking whether the name you have given to an alkane is reasonable is to count the number of carbon atoms implied by the chosen name. If you were to check the given structure and find 11 carbon atoms, you would know that you had made a mistake. When naming alkanes, a common error of beginning students is a failure to pick out the longest carbon chain. Remember that every substituent must have a number, and do not forget the prefixes: di, tri, tetra, etc. You must use commas to separate numbers, and hyphens to separate numbers and substituents. Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes , depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds.
Sonata mens watch lowest price
The compound has a double carbon-carbon bond, therefore it is an alkene and the suffix is -ene. F comes before i 1-fluoro-2,2-diiodo. The -ane tells us there are only single carbon-carbon bonds. Note that the methyl and iodo are written in alphabetical order. The organic compound therefore contains '1,3-diene'. There is a halogen atom and only single carbon-carbon bonds, therefore this is a haloalkane and the suffix is -ane. The double bond is given the lowest possible number and so this compound is not propene. The suffix yne means that this compound is an alkyne and there must be a triple bond located on carbon number 3. There are two carbon atoms in the longest chain, therefore the prefix is ethan-. There are three carbon atoms in the longest chain. Summary of functional groups. This playdough can be used for all the model building activities. The prefix but- tells us there are four carbon atoms in the longest chain containing the functional group. Functional group.
We will only use those common names listed under Objective 3, above. We will use systematic names in all other cases. For example, the systematic name of the compound shown below is benzenecarbaldehyde, but it has the common name of benzaldehyde.
We must number the chain so that the carbonyl group is always the first carbon atom. The prefix propan- tells us there are three carbon atoms in the longest chain and only single carbon-carbon bonds. Combine the elements of the compound's name into a single word in the order of branched groups; prefix, name ending according to the functional group The compound's name is butanone or 2-butanone. There are four carbon atoms in the longest chain so the prefix is but-. Draw the structural and condensed structural formula for the organic compound 2-iodomethylpentane. There is a triple bond between two of the carbon atoms, so this compound is an alkyne. It also enables every compound to have a unique name, which is not possible with the common names used for example in industry. There is a branched group on carbon 3. If there is more than one double bond, the suffix is expanded to include a prefix that indicates the number of double bonds present -adiene , -atriene , etc. If we start at the carbon on the left, we can number the atoms as shown in red left. This is therefore an ester and the suffix is -oate. Name the halogen atom and assign the number for the carbon atom it is attached to The halogen is a chlorine atom.

It is necessary to be the optimist.
It is unexpectedness!