Which of the following is an example of homogeneous mixture
Key Points.
The correct answer is Salt solution. Key Points. Additional Information Homogeneous mixture. Last updated on Nov 8, Bihar police Constable Notification will be released soon. A total of Vacancies is expected to be announced for the post of Bihar police constable. The written test was held on 2nd, 3rd, 9th, 10th, 16th, 23rd, and 30th December for Bihar police Constable
Which of the following is an example of homogeneous mixture
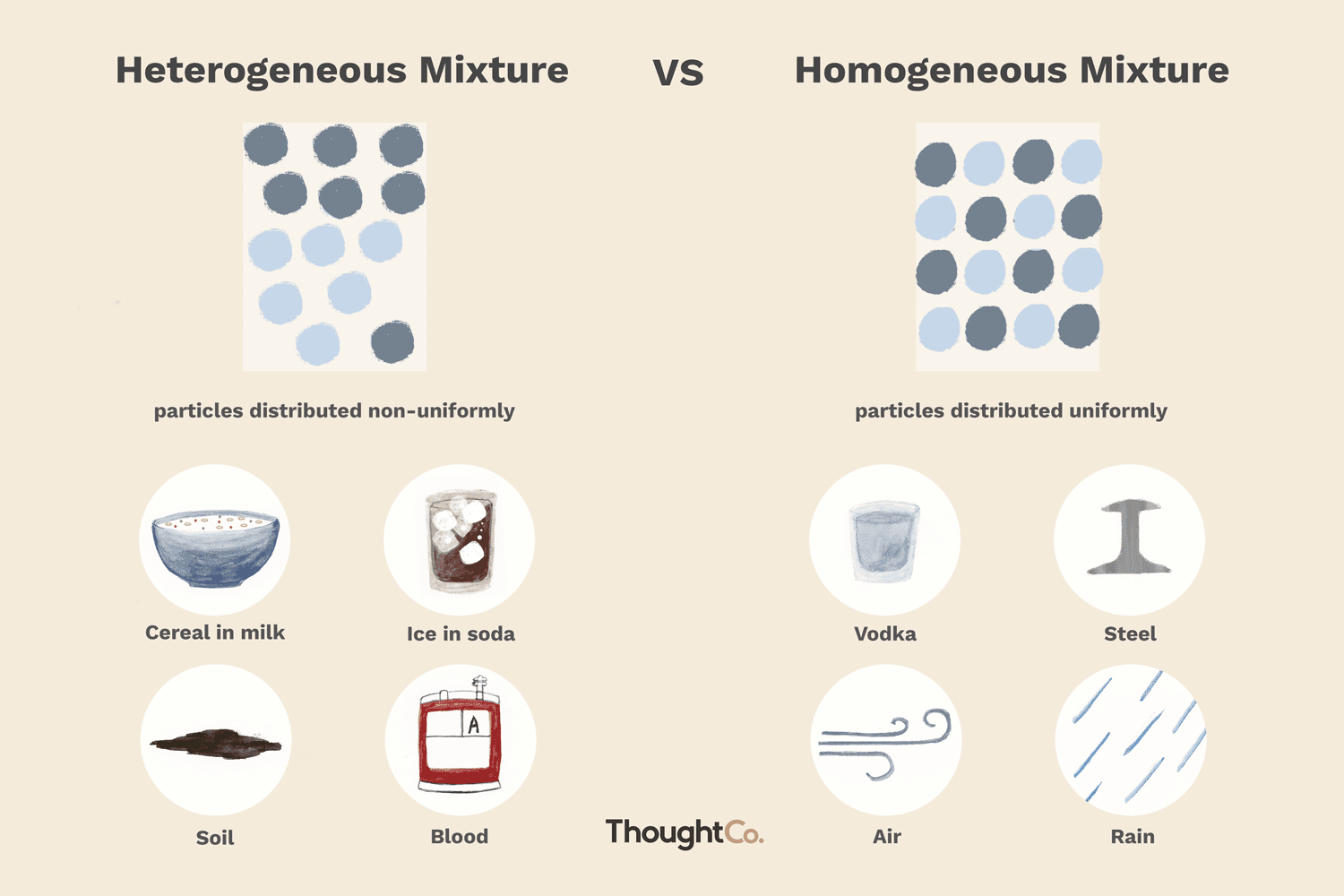
When you combine two or more materials, you form a mixture. In chemistry, a mixture is a combination that does not produce a chemical reaction. There are two categories of mixtures: homogeneous mixtures and heterogeneous mixtures. Here's a closer look at these types of mixtures and examples of mixtures. Homogeneous mixtures appear uniform to the eye. They consist of a single phase, be it liquid, gas, or solid, no matter where you sample them or how closely you examine them. The chemical composition is the same for any sample of the mixture. Heterogeneous mixtures are not uniform. If you take two samples from different parts of the mixture, they will not have an identical composition. You can use a mechanical method to separate components of a heterogeneous mixture e. Sometimes these mixtures are obvious, where you can see different types of materials in a sample. For example, if you have a salad, you can see different sizes and shapes and types of vegetables. In other cases, you need to look more closely to recognize this mixture. Any mixture that contains more than one phase of matter is a heterogeneous mixture. This can be tricky because a change of conditions can alter a mixture.
Maharashtra Police Constable.
.
Many people enjoy a cup of coffee at some point during the day. Some may drink it black, while others may put cream or some dairy substitute and sugar in their coffee. You can buy high-end coffee drinks at espresso stands either sit-down or drive-through. Whatever your preference, you want the coffee to be the same at the beginning and the end of your drink. Ordinary table salt is called sodium chloride. It is considered a substance because it has a uniform and definite composition.
Which of the following is an example of homogeneous mixture
The major component of a solution is called the solvent. The minor component of a solution is called the solute. By major and minor we mean whichever component has the greater or lesser presence by mass or by moles. Sometimes this becomes confusing, especially with substances with very different molar masses. However, here we will confine the discussion to solutions for which the major component and the minor component are obvious. Solutions exist for every possible phase of the solute and the solvent. In all cases, however, the overall phase of the solution is the same phase as the solvent. A solution is made by dissolving 1.
Convert 20 pounds to kilograms
Gujarat Metro Maintainer. CG Forest Guard. Rajasthan Gram Vikas Adhikari. JK Police Constable. OFDC Assistant. Maharashtra State Excise Jawan. Punjab Police. CG Vyapam Assistant Grade 3. Ananya Singh. Police Exams. Understand audiences through statistics or combinations of data from different sources. IB Security Assistant. It can be distinguished after they are mixed. Delhi Police Head Constable. Delhi Police MTS.
The two types of mixtures are homogeneous mixtures and heterogeneous mixtures. Here are 10 examples of mixtures and a look at whether they are homogeneous or heterogeneous. A homogeneous mixture is one which appears to have uniform composition.
RBI Office Attendant. English Hindi. GATE Chemistry. Rajasthan Home Guard. Vizag Steel Management Trainee. Maharashtra Zilla Parishad Extension Officer. Delhi Forest Guard. CG Vyapam Sub Engineer. Rajasthan High Court System Assistant. Telangana High Court Field Assistant.

In my opinion you are mistaken. I can prove it. Write to me in PM, we will talk.
Bravo, remarkable idea
Yes, you have truly told