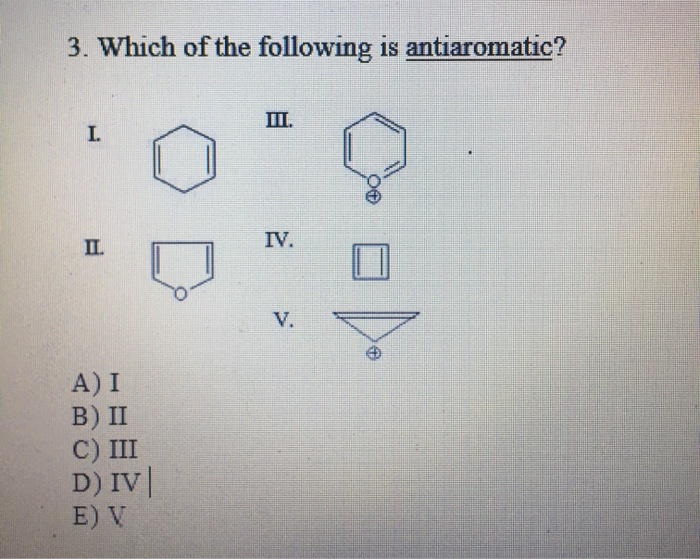
Which of the following is antiaromatic
In bt21 imagenes to the diamagnetic ring current present in aromatic compoundswhich of the following is antiaromatic, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene Abiphenylene Bcyclopentadienyl cation C. The prototypical example of antiaromaticity, cyclobutadieneis the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing to its destabilization.
Hence, is anti-aromatic. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
Which of the following is antiaromatic
Which of the following is an antiaromatic compound? Which of the following species are antiaromatic? Which of the following are pairs of antiaromatic species? Which of the following species is antiaromatic? Consider the following reactions : Which of the following are stereospecific reactions? Antidepressant drugs follow which of the following mechanism? Sulphur, on heating follows Which of the following sequence? Match the following: Which of the following is the best matched options? Which of the followig is aromatic? Which of the following is not aromatic? Which of the followng is the least reactive towards addition reactions The order of stability of the species i , ii and iii is. In which of the following compounds are the hydrogen atoms atoms of th
View Solution. WB Police.
.
In short, the only way aromatic and antiaromatic compounds differ is the number of electrons they have in the conjugated system. All the other criteria — being cyclic, planar, and fully conjugated are a must for both categories:. There is also the third class of compounds we need to discuss: These are the nonaromatic or not aromatic compounds. As the name suggests, nonaromatic compounds have really nothing to do with the aromaticity well, almost. And that is the idea, nonaromatic compounds are the ones not related to the aromatic and antiaromatic compounds:. In fact, if any of these factors; cyclic, planar, fully conjugated does not match — the compound is said to be nonaromatic. Sometimes though, it may not be so obvious because some molecules look like they are aromatic or antiaromatic based on the factors mentioned above. A good example is cyclooctatetraene which we have talked about earlier :. It looks like a perfect candidate to be named antiaromatic — cyclic, planar, fully conjugated, and 8 electrons 4n formula for antiaromatic compounds.
Which of the following is antiaromatic
Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems. The Pi Molecular Orbitals of Benzene. It is similar to the requirements for aromaticity, except for one key factor in red.
هدى المفتي
Indian Army Agniveer. Rajasthan Housing Board JE. TS SET. SSB Head Constable. Bihar Police Forest Guard. BEL Senior Engineer. MP Vyapam Sub Engineer. Cyclooctatetraene is another example of a molecule which is not antiaromatic, even though it might initially appear to be so. SBI SO. NCL Staff Nurse. The term PAN in chemical refers to. Navy Tradesman Mate. Madras High Court Office Assistant. CMAT Exam. A negative NICS value is indicative of aromaticity and a positive value is indicative of antiaromaticity.
Some Practice Problems.
UP Police Workshop Staff. Maharashtra Agricultural Officer. The chemical shift for the protons outside its ring is 5. The term 'antiaromaticity' was first proposed by Ronald Breslow in as "a situation in which a cyclic delocalisation of electrons is destabilising". Bihar D. EMRS Driver. Maharashtra Zilla Parishad Rigman. UP Police. Journal of the American Chemical Society. Goa Police Constable. Gujarat Forest Guard. Indian Army Nursing Assistant.

Quite right! I think, what is it good idea.