Which of these is an extensive property of a substance
One of the ways we can describe chemical substances is with extensive and intensive properties. This video will teach you about the difference between these two terms. You will also see some examples of each, and you'll have a chance to practice what you've learned at the end of the video.
You agree to mow someone's lawn for twenty dollars it's a fairly large yard. Some properties of matter depend on the size of the sample, while some do not. An extensive property is a property that depends on the amount of matter in a sample. The mass of an object is a measure of the amount of matter that an object contains. A small sample of a certain type of matter will have a small mass, while a larger sample will have a greater mass. Another extensive property is volume.
Which of these is an extensive property of a substance
Physical or chemical properties of materials and systems can often be categorized as being either intensive or extensive , according to how the property changes when the size or extent of the system changes. The terms "intensive and extensive quantities" were introduced into physics by German mathematician Georg Helm in , and by American physicist and chemist Richard C. Tolman in By contrast, an extensive property or extensive quantity is one whose magnitude is additive for subsystems. Not all properties of matter fall into these two categories. For example, the square root of the volume is neither intensive nor extensive. An intensive property is a physical quantity whose value does not depend on the amount of substance which was measured. The most obvious intensive quantities are ratios of extensive quantities. In a homogeneous system divided into two halves, all its extensive properties, in particular its volume and its mass, are divided into two halves. All its intensive properties, such as the mass per volume mass density or volume per mass specific volume , must remain the same in each half.
For example, a volume transfer is associated with a change in pressure. Chemistry Introduction to Chemistry Concepts and Terms. On the Equilibrium of Heterogeneous Substances.
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition the identities of the substances contained in the matter. We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water Figure 1.
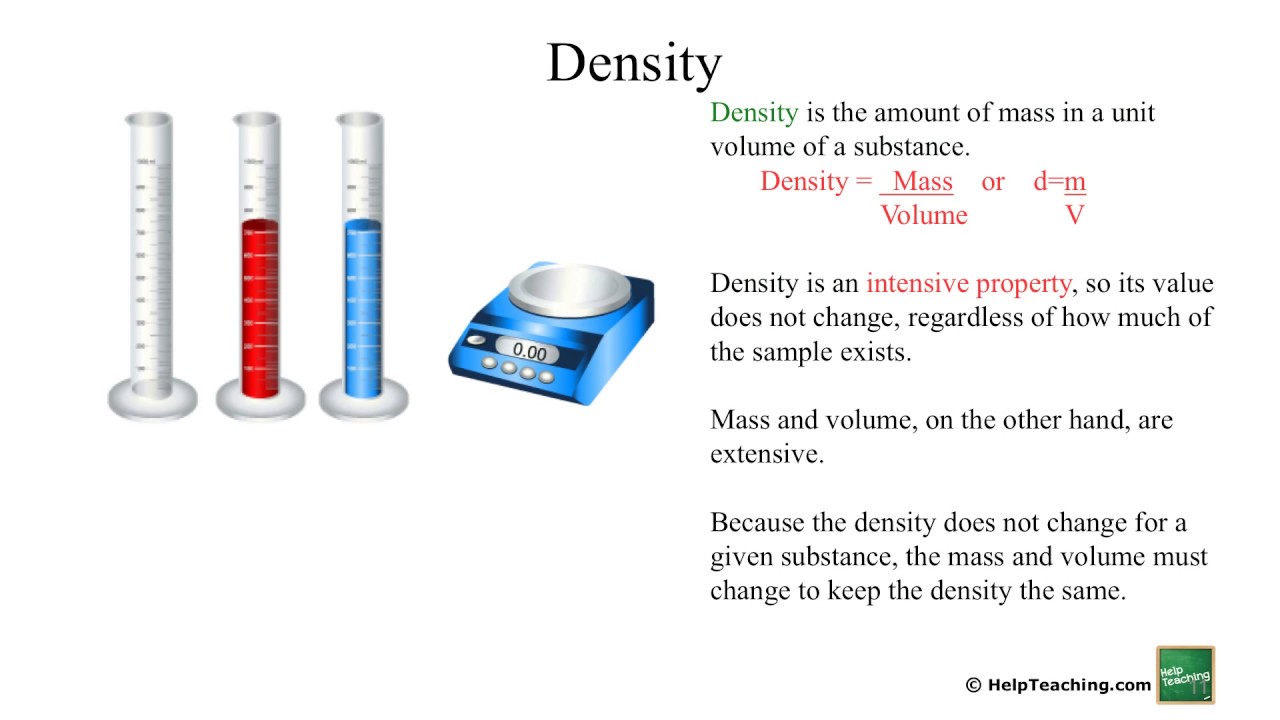
The two types of physical properties of matter are intensive properties and extensive properties. Here is the definition of an extensive property in chemistry. An extensive property is a property of matter that changes as the amount of matter changes. Like other physical properties, an extensive property may be observed and measured without any chemical change reaction occurring. Mass and volume are extensive properties. As more matter is added to a system, both mass and volume changes. In contrast to extensive properties, intensive properties do not depend on the amount of matter in a sample.
Which of these is an extensive property of a substance
The characteristics that distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. Some physical properties, such as density and color, may be observed without changing the physical state of the matter. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in the chemical identities of the substances contained in the matter.
Carros dodge 300 en venta
System properties Note: Conjugate variables in italics Property diagrams Intensive and extensive properties. The same milk is in each container. Other examples of chemical changes include reactions that are performed in a lab such as copper reacting with nitric acid , all forms of combustion burning , and food being cooked, digested, or rotting Figure 3. Define intensive property. If the property of a sample of matter does not depend on the amount of matter present, it is an intensive property. Toggle limited content width. Let's consider a diamond. The most obvious intensive quantities are ratios of extensive quantities. Exercises Classify the six underlined properties in the following paragraph as chemical or physical: Fluorine is a pale yellow gas that reacts with most substances. Review Define extensive property. Specific internal energy.
All matter has physical and chemical properties.
Being extensive properties, both mass and volume are directly proportional to the amount of substance under study. Licenses and Attributions. Give two examples of intensive properties. They are transferred across a wall between two thermodynamic systems or subsystems. Specific enthalpy. Online corrected version: — " Intensive quantity ". Bibcode : JChEd.. These properties can be used to sort the elements into three classes: metals elements that conduct well , nonmetals elements that conduct poorly , and metalloids elements that have properties of both metals and nonmetals. Hazard Diamond You may have seen the symbol shown in Figure 4 on containers of chemicals in a laboratory or workplace. Give two examples of extensive properties. Let's consider our diamond again. An entropy change is associated with a temperature change. What Is an Element? It follows, for example, that the ratio of two extensive properties is an intensive property. The temperature of a system in thermal equilibrium is the same as the temperature of any part of it, so temperature is an intensive quantity.

I suggest you to visit a site on which there are many articles on a theme interesting you.
I hope, you will come to the correct decision. Do not despair.