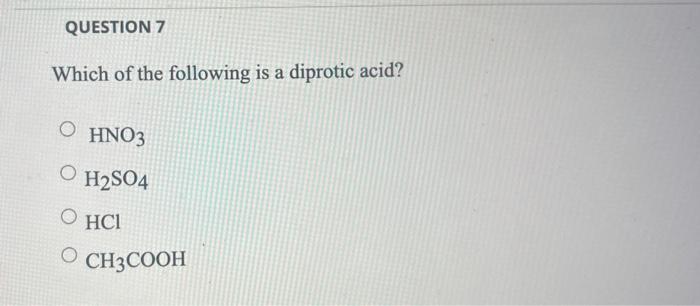
Which one of the following is a diprotic acid
The acid equilibrium problems discussed so far have focused on a family of compounds known as monoprotic acids. There is usually a large difference in the ease with which these acids lose the first and second or second and third protons. When sulfuric acid is classified as a strong acid, students often assume that it loses both of its protons when it reacts with water. That isn't a legitimate assumption.
Additional Information. Acetic acid is monoprotic acid due to its structure. The three hydrogen atoms attached to the carbon are in weak polar bonds. They do not ionize. Only the H bonded to the highly electronegative oxygen can be ionized. Last updated on Mar 31,
Which one of the following is a diprotic acid
.
Supreme Court Law Clerk. ICAR Technician.
.
Definition: A diprotic acid is an acid that can donate two proton or hydrogen atom per molecule to an aqueous solution. Compare this to a monoprotic acid. Examples: Sulfuric acid H 2 SO 4 is a diprotic acid. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance.
Which one of the following is a diprotic acid
Acids are classified by the number of protons per molecule that they can give up in a reaction. Their reactions with water are:. Even though it contains four hydrogen atoms, acetic acid, CH 3 CO 2 H, is also monoprotic because only the hydrogen atom from the carboxyl group COOH reacts with bases:. Diprotic acids contain two ionizable hydrogen atoms per molecule; ionization of such acids occurs in two steps. The first ionization always takes place to a greater extent than the second ionization. For example, sulfuric acid, a strong acid, ionizes as follows:. This stepwise ionization process occurs for all polyprotic acids. Carbonic acid, H 2 CO 3 , is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts.
Redcafe
Bihar Police Fire Station Officer. Mazagon Dock Shipbuilders Non Executive. AP Animal Husbandry Assistant. BEL Senior Engineer. TS TET. We now solve this approximate equation for C. Allahabad High Court RO. If our assumptions so far are correct, the HPO 4 2- ion concentration in this solution is equal to K a2. HPCL Engineer. Rajasthan Gram Vikas Adhikari. ICMR Assistant. CG TET. Bihar AMIN. WB Police SI. What is another name of quick lime?
The acid equilibrium problems discussed so far have focused on a family of compounds known as monoprotic acids.
Bihar CET B. Bihar Senior Secondary Teacher. Gujarat Metro. Bihar Vidhan Sabha Junior Clerk. Bihar Police Fire Station Officer. Rajasthan Pre D. H 2 S is a weak acid that dissociates in steps. Which is the most abundant acid found in grapes? BPSC Exam. Telangana Police SI. CAT Exam.

We can find out it?
Excuse for that I interfere � I understand this question. Let's discuss. Write here or in PM.
I can not participate now in discussion - it is very occupied. I will be released - I will necessarily express the opinion on this question.