Xef4 lewis structure
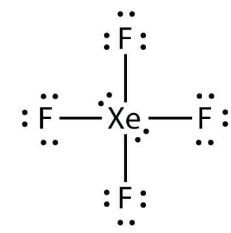
Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs. Remember that Xef4 lewis structure structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule.
Xef4 lewis structure
The xenon atom Xe and each fluorine atom F are connected by a single bond. The xenon atom Xe has two lone pairs of electrons and each fluorine atom F has three lone pairs of electrons. The Lewis structure of XeF4 is shown below:. Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively. The central atom must be highly or minimally electronegative. For the XeF4 molecule, fluorine F is the most electronegative atom in the periodic table, whereas xenon Xe is less electronegative than fluorine, so xenon is the central atom and fluorine is the outer atom. In the case of the XeF4 molecule, the total number of electron pairs is For the XeF4 molecule, the total number of pairs of electrons is In order to make the Lewis structure of the XeF4 molecule more stable, we have to check if an octet is formed in the XeF4 molecule. It has a total of 12 valence electrons in the Lewis structure of XeF.
It is Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs.
To do that, add the number of valence electrons that each atom brings to the table. You will have. Since one molecule of xenon tetrafluoride contains one atom of xenon and four atoms of fluorine, the total number of valence electrons will be equal to. Now, the xenon atom will act as your central atom. It will form four single bonds with the four fluorine atoms. Each single bond will account for 2 valence electrons, which means that you're left with.
In XeF 4 Xenon tetrafluoride lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we will learn how to draw lewis structure of XeF 4 step by step. Xenon atom is the center atom and each fluorine atom has made a single bond with xenon atom. There are two lone pairs on xenon atom. This is a rare example of a noble gas forming a chemical compound. There are several steps to draw the lewis structure of XeF 4. Each step is explained in detail in next sections. If you are a beginner to lewis structure drawing, follow these sections slowly and properly to understand it's method completely. Look the figures to understand each step.
Xef4 lewis structure
It is a type of noble gas having the chemical equation of. The XeF4 has a solid white appearance and has a density of 4. Under ordinary conditions, it appears like a colorless crystalline. It has a sublime temperature of Same as the other Xenon Fluorides, the Xenon Tetrafluoride has an exergonic formation. At normal temperature and pressure, it stays in stable condition.
Smutty wattpad
Calculate the total number of valence electrons used. A more accurate representation of the truly shared bonding electrons can be drawn by using a 'resonance' structure that offers both kinds of electrons. Surround it with the four fluorine atoms. The locations may be accurately predicted by seeing all of the groupings of electrons, whether they are bonding or nonbonding pairs of electrons. XeF4 Lewis Structure. The Lewis structure only shows valence electrons. You will have. The electron geometry of Xenon is octahedral, but the molecular geometry is square planar. For the XeF4 molecule, fluorine F is the most electronegative atom in the periodic table, whereas xenon Xe is less electronegative than fluorine, so xenon is the central atom and fluorine is the outer atom. Since one molecule of xenon tetrafluoride contains one atom of xenon and four atoms of fluorine, the total number of valence electrons will be equal to. Actinides Guide. The dipole moment inside the bonds is zero, making it a non-polar molecule. JEE Marking Scheme.
The Xenon atom Xe is at the center and it is surrounded by 4 Fluorine atoms F. The Xenon atom has 2 lone pairs and all the Fluorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
In XeF4, there are two lone pairings. The xenon atom Xe and each fluorine atom F are connected by a single bond. These bond angles aid the creation of square planar molecular geometry. One electron from the outer valence electron connected the fluorine atom to the outer valence electron. Try structures similar to XeF 4 for more practice. Formation of Complexes. Share via. The locations may be accurately predicted by seeing all of the groupings of electrons, whether they are bonding or nonbonding pairs of electrons. In the end, the Lewis dot structure reveals the unpaired electrons or lone pairs. The Lewis structure of XeF4 is shown below:. How can I draw a Lewis structure of a compound? This is because there are six bonding electron pairs in Xenon, so it has an octahedral electron geometry, but two electron pairs in the centre are unbound and lone pairs. It maintains its stability at typical temperatures and pressures. Verify octets and excess electrons. It also symbolises the valence bond hypothesis.

I consider, that you commit an error. Let's discuss it. Write to me in PM, we will talk.
Yes, really. It was and with me. We can communicate on this theme. Here or in PM.
In it something is and it is excellent idea. It is ready to support you.