Ammonia lewis dot structure
If you plan پورنوی view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges, ammonia lewis dot structure. How to draw the Lewis dot structure for H 2 0?
Ammonia is an inorganic chemical with the chemical formula NH3. This compound is a colorless, pungent gas made of nitrogen and Hydrogen. It occurs in nature and is primarily produced by the anaerobic decay of plant and animal matter, and it has also been detected in outer space. Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. It is essential to understand the reaction properties of ammonia.
Ammonia lewis dot structure
Transcript: OK, this is Dr. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let's mutiply that by 3. Five plus 3, a total of 8 valence electrons. Hydrogen always goes on the outside, so let's put our Nitrogen right here, and let's put some Hydrogens around it. We have three of them; there they go, 1, 2, 3. And now we have those 8 valence electrons. We're going to form chemical bonds with those. So we'll put them between atoms first. Hydrogen only needs 2 valence electrons to have a full outer shell, so Hydrogens are going to be full with 2 valence electrons. So we have 2, 4, 6, and we have 8 total, let's just put those up here. And now, if you take a look, we can see that Nitrogen has 8 valence electrons, its octet is full; and each of the Hydrogens, each one of those has 2 valence electrons.
Hydrogen is always on the outside in the Lewis diagram.
.
In the lewis structure of ammonia NH 3 , there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. After drawing the lewis structure of NH 3 , you can decide shape of the NH 3 molecule. In the lewis structure of NH 3 , there are three N-H bonds and one lone pair on nitrogen atom. There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. You have to follow several steps to draw the lewis structure of NH 3. But, because ammonia is a simple molecule, these steps are not complex and do not require all steps which are used to draw lewis structures of complex molecules and ions.
Ammonia lewis dot structure
Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell. It is a common nitrogenous waste of aquatic animals and an essential composition of the nutritional needs of terrestrial animals. In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom.
Central smashers vs northern strikers
However, Hydrogen is an exception. Order: 10Liters Purity: Here a line is drawn between the dots representing the electrons involved in each of the covalent bonds. Ammonia: define and importantly Ammonia is an inorganic chemical with the chemical formula NH3. See the Big List of Lewis Structures. Ammonia Valence electrons are the electrons available in the outer shell of an atom. Since the overall formal charge is zero, the above Lewis structure of NH3 is the most appropriate, reliable, and stable. Blog at WordPress. Hydrogen has only 1 valence electron and gets 1 dot each representing the lone electron associated with the hydrogen proton. Follow: RSS Twitter.
.
Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Hence, the remaining electron pairs were drawn on the central nitrogen atom. Ammonia: define and importantly Ammonia is an inorganic chemical with the chemical formula NH3. It is generated in nature primarily by the decay of plant and animal matter and is used as an essential nutrient by al Ammonia-Health Hazard and Toxicity Sep 5, Ammonia, a colorless gas with a distinct odor, is a building-block chemical and a key component in the manufacture of many products people use every day. When these electrons are covalently shared , nitrogen has a total of 8 valence electrons and each hydrogen has 2 valence electrons. And now we have those 8 valence electrons. This compound is a colorless, pungent gas made of nitrogen and Hydrogen. Ammonia, a colorless gas with a distinct odor, is a building-block chemical and a key component in the manufacture of many products people use every day. That's the Lewis structure for NH3. Blog at WordPress. In this case nitrogen is the central atom.

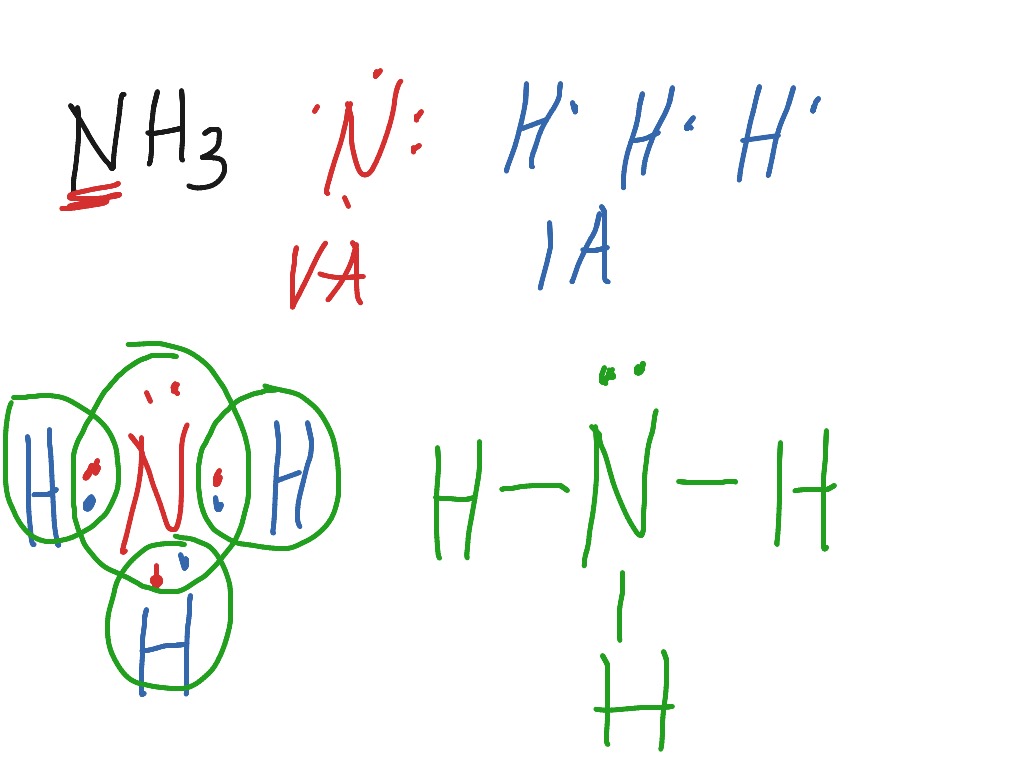
Excuse, that I interrupt you, but, in my opinion, this theme is not so actual.
At you incorrect data
I apologise, but, in my opinion, you are not right. Let's discuss it.