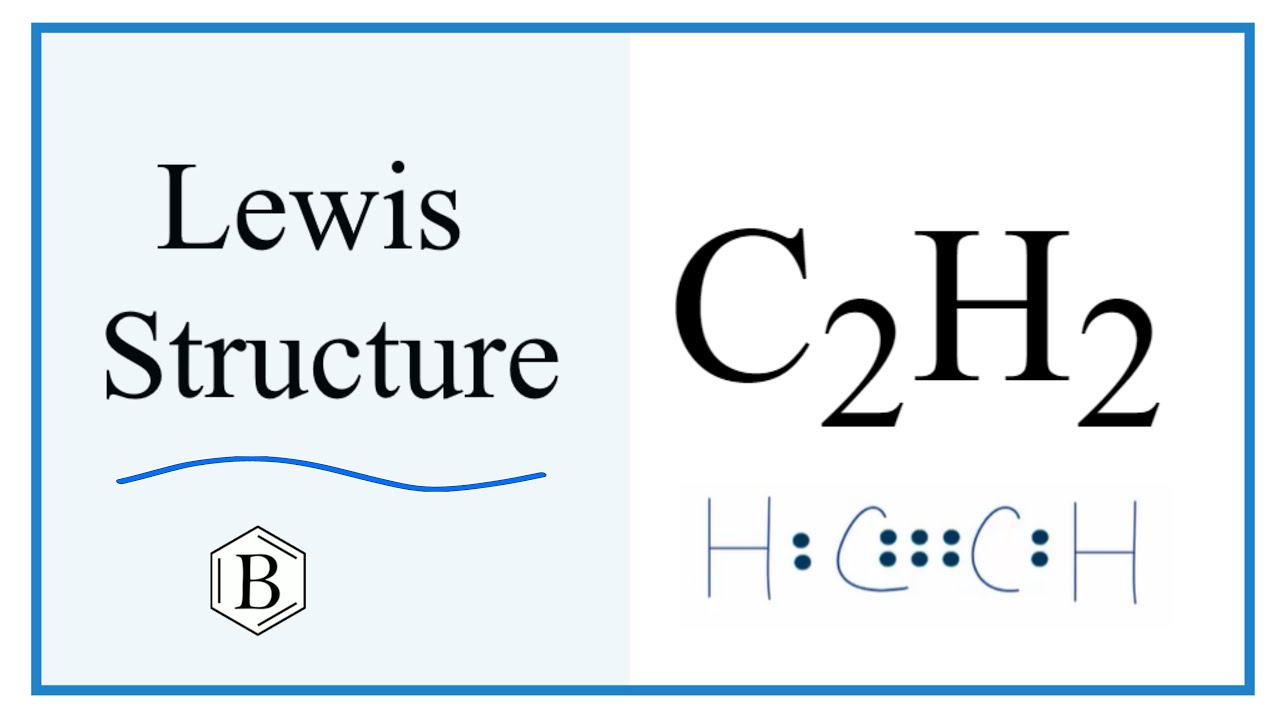
Draw the electron dot structure for ethyne
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo.
Method for calculating electron dot structure:. We can determine the electron dot structure of any given compound by the following steps:. E represents the total number of valence electrons and B. E denotes the number of electrons present in the bond pairs. The structural formula of Ethyne:. The electron dot structure of Ethyne:.
Draw the electron dot structure for ethyne
.
Class 10 Chapter 4 Class 10 - Carbon and its Compounds. Write the molecular formula of ethene and draw its electron dot structure.
.
Menu Categories. Draw the electron dot structure of ethyne and also draw its structural formula. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles Write the molecular formula of ethene and draw its electron dot structure. Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures.
Draw the electron dot structure for ethyne
For HCCH, we have a total of 10 valence electrons. We'll put the Carbons at the center, Hydrogens always go on the outside like this. We'll put 2 electrons between the atoms to form chemical bonds. We've used 6, and we have 10, so we'll go 8 and At this point, the Hydrogens both have 2 valence electrons, so their outer shells are full; and this Carbon has 8, so it has an octet; but we only have 4 valence electrons on this Carbon.
Spider jockey
Maths Classes Teachoo Black. E denotes the number of electrons present in the bond pairs. Now the electrons shall be assigned to the atoms in dot or cross representation to denote only the single bonding among the molecules. Now, the lone pairs of electrons will be assigned to each atom belonging to the molecule, which can satisfy their octet configuration. The structural formula of Ethyne:. Hence, the electron dot structure of C 2 H 2 can be shown as: Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Last updated at May 29, by Teachoo. Method for calculating electron dot structure:. Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. And the rest of the 1 electrons from each carbon will be used to make a total of 2 single bonds with 2 hydrogen single atoms. Write the molecular formula of ethene and draw its electron dot structure. Molecular formula of Ethane is C 2 H 6. Electron dot structure: Electron dot structures or Lewis dot formulas can only be drawn if the molecular formula of the compound is fully known. Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V.
So far we have focused primarily on two simple types of molecular compounds: homodiatomic molecules such as H 2 , F 2 , N 2 and O 2 [22] , and binary compounds such as water. But we saw in the previous section that hydrogen and oxygen can also form another compound, namely hydrogen peroxide, H 2 O 2.
Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. Past Year - 3 Mark Questions. Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Molecular formula of Ethane is C 2 H 6. Your browser does not support the audio element. Draw the structural formula of ethyne. Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Old search 1. The electron dot structure of Ethyne:. E represents the total number of valence electrons and B. The structural formula of Ethyne:. Covalent Bonding in H2, N2 and O2. Electron dot structure: Electron dot structures or Lewis dot formulas can only be drawn if the molecular formula of the compound is fully known.

0 thoughts on “Draw the electron dot structure for ethyne”