Hbr lewis structure
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br, hbr lewis structure.
HBr is the chemical formula of hydrogen bromide. We are learning here about HBr lewis structure, characterizations and quick facts. Hydrogen bromide is an anhydrous gas with no colour having strong irritating smell. It is corrosive in nature and heavier than air. HBr molecule contains one hydrogen atom and one bromine atom in its structure. The molecular weight of HBr is HBr has synonyms like bromane, hydrobromic acid, hydrobromide, etc.
Hbr lewis structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms.
So, there is no possible movement of electrons in HBr lewis structure to form multiple bonds. In order to check the stability of the central bromine Br atom, we have to check whether it hbr lewis structure forming an octet or not. Why HBr is unstable?
.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms.
Hbr lewis structure
Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways. It is used to prepare a variety of organic and inorganic bromine compounds. Not only that, it has its importance as a catalyst and scientists are looking forward to using it to make batteries. Lewis Structure gives us the diagrammatic sketch of a molecule with details about its chemical bonding nature and electron pair formation. If we are interested in having an idea about the internal structure and nature of a given molecule, we need to learn about the sigma pi bond formation and also about lone pairs. Also known as electron dot structure, we use Lewis symbols so that we can find out the electronic configuration of the inside atoms of a molecule or ions.
Scorpio-sagittarius cusp man
Is HBr diprotic? Hence, HBr is monoprotic molecule. Pure form of HBr hydrogen bromide is present in gaseous form. Thus due to intermolecular forces like hydrogen bonds with water hydrobromic acid is viscous in nature. Hydrogen bromide HBr is diamagnetic in nature, due to the presence of all paired electrons in the molecule. So, the HBr lewis structure has one central H atom, one bonding Br atom and three lone electron pairs on Br atom. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom H and bromine atom Br. It can accept electrons. Hence, there is the formation of strong intermolecular forces between H and Br atoms of HBr molecules i. How HBr is amphiprotic? Thus the electronegativity difference between H and Br atoms of HBr molecule is 0.
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide HBr. It showcases the connectivity between hydrogen H and bromine Br atoms, giving us a visual representation of their covalent bond. Determine the Total Valence Electrons.
But HBr hydrobromic acid cannot accept protons when reacts with base. Organic compounds are the compounds which contains carbon atom in its molecule or structure. The HBr molecule composed of H and Br atoms which have 2. How HBr is conductive? Is HBr balanced? Electrolyte is a substance which when dissolve in water get ionizes and conduct electricity. Here, HBr behaves a s electrophile because it can accepts electron pair from ethane and donated its hydrogen atom or proton and creates a C-H bond with ethane molecule and the Br ion also attached with another carbon atom of ethane. Is HBr viscous? Why HBr an Arrhenius acid? This single covalent bond is quite strong bond which is not easily gets break. Both H and Br atoms thus share one — one electron with each other to form a single covalent bond. Covalent molecules are those which have electronegativity difference value of less than 0. HBr is non — metallic. No, HBr is not neutral molecule. The HBr has linear shape and tetrahedral geometry with sp3 hybridization and

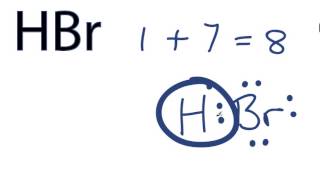
Now all became clear, many thanks for the information. You have very much helped me.