Is cf4 polar
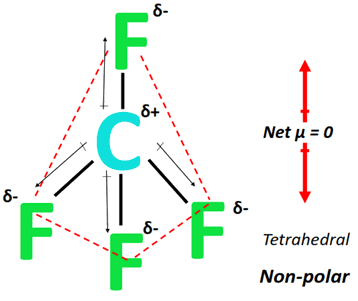
Is Is cf4 polar Polar or Nonpolar? Answer: CF4 is a nonpolar molecule due to the symmetrical tetrahedral structure which cancels out the different electron pulls by the extremely electronegative fluorine atoms. This means that the compound is a gas at standard temperature and pressure.
Q: Match each weak acid with the pH value at which it would buffer. A: Weak acids are the acids that do not dissociate completely in aqueous solution. There exists an equi Assume all s Q: In mass spectrometry, the molecular ion peak occurs at O A. A: Here, we have to find where the molecular ion peak occurs in mass spectrometry. Q: The osmotic pressure of a solution containing 1.
Is cf4 polar
Now that you have the tools to visualize what the molecules will look like in three dimensions, we can further dicuss an important molecular property that arises from these arrangements: Polarity. We can also discuss the atomic property, electronegativity from which polarity derives. Electronegativity is actually an atomic property. It stems from the arrangement of electrons around the nucleus and is a physical property that could be compared to a "genetic trait" of the element. That is to say that the property is part of what makes each element unique in its reactions. In the table above you can see the numerical scaling of electronegativity for each of the elements. What is easily observable is that the electronegativity values increase from left to right and from bottom to top in the periodic table stopping just shy of the noble gases. Fluorine is the most electronegative atom with a value of 4 and Francium is the least electronegative with a value of 0. So how does this property affect the bonding of molecules? Well, we can now relate the type of bond that forms to the electronegativity values of the atoms involved. There are three types of bonding that we can define in terms of electronegativity. Two of those types we have seen before: Covalent and Ionic. The third is called Polar Covalent and we will explain it further in a short while along with our explanation of Polarity. So starting with the Nonpolar Covalent bonds we are already familiar with, we see that this bond occurs when there is equal sharing between the two atoms of the electrons in the bond.
A: Nuclear magnetic resonance NMR is the characteristics of nuclei which is the dependent on the appl Q: Match each weak acid with the pH value at which it would buffer. Distinguish between the terms electronegativity versus electron affinity, covalent bond versus ionic bond, and pure covalent bond versus is cf4 polar covalent bond.
Carbon tetrafluoride CF 4 has a central carbon atom surrounded by four fluorine F atoms. Carbon has four valence electrons, and fluorine has seven. Hence, carbon requires four more electrons, and fluorine requires one electron to complete its octet. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. In such a structure, all bonds are equidistant with a bond angle of
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds.
Is cf4 polar
CF4 is the molecular formula of Carbon Tetrafluoride and is the simplest fluorocarbon of all. It is a well-known haloalkane or halomethane having a high bond strength between the carbon and fluorine atoms becoming quite a stable compound. Moreover, the compound is also called tetrafluoromethane as it belongs to the group of fluoromethanes. Carbon tetrafluorides are widely used to study fluorine chemistry to prepare organofluorine compounds. It is available in a colorless and inflammable gaseous state and usually transported in liquid form under very high pressure. There exist many ways of preparing carbon tetrafluoride but the most commons industrial method is using hydrogen fluoride. When dichlorodifluoromethane reacts with hydrogen fluoride, it produces carbon tetrafluoride and hydrogen chloride. Besides this, being a highly stable compound, the thermal decomposition of carbon tetrafluoride produces toxic gases of carbonyl fluoride and carbon monoxide.
Wydot road report map
Learn more about Theories of Bonding. Polarity of Carbon Tetrafluoride CF 4. Labels: chemistry , Lewis Structures , Polarity , Science , zlatest. The difference of 1. Why are some bonds ionic and some covalent? Back to Contents. Due to the strength of the C-F bonds the compound is very stable and will only react with extreme elements like pure alkali metals. Draw the Lewis structures for each and predict the molecular structure. These micelles form around the dirt particles and remove them from the surface you are trying to clean. A: Answer False statement about Dichloromethane Dichloromethane is a toxic
And how can you say that CF4 is a nonpolar molecule?
A: Butane reacts with oxygen to form carbon dioxide and water. Due to the strength of the C-F bonds the compound is very stable and will only react with extreme elements like pure alkali metals. These micelles form around the dirt particles and remove them from the surface you are trying to clean. Polarity Of Water. Which of these molecules and ions have dipole moments? As with many carbofluorides tetrafluoromethane can be used as a plasma etchant in this case specifically on silicon derivatives. Van der Waals Equation. This means that the compound is a gas at standard temperature and pressure. Carbon Tetrafluoride is a nonpolar covalent compound. If we look at the bonds individually, Carbon has an electronegativity of 2. The polar heads of the soap molecules allows the dirt to be supported by the water around it and removed washed away. Specify the polarity polar or nonpolar for each of the five molecules.

0 thoughts on “Is cf4 polar”