Lewis dot diagram for c2h6
In order to find the total valence electrons in C2H6 moleculefirst of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer. Open in App. Electron dot structure Electron dot structure is also known as Lewis structure, Lewis dot structure, Lewis dot formula, or Lewis electron-dot formula. It is a way of representing atoms, ions, or molecules by showing the outermost shell electrons as dots surrounding the element symbol.
Lewis dot diagram for c2h6
Transcript: Hi, this is Dr. Let's do the Lewis structure for C2H6, ethane. On the periodic table, Carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. So let's multiply that times 2. And then Hydrogen, group 1, one valence electron; we have 6, multiply that by 6, for a total of 14 valence electrons to work with. Hydrogen always goes on the outside, so we'll draw our Carbons. And then we'll put the six Hydrogens: 1, 2, 3, 4, 5, 6 Hydrogens around there. So we're going to take and put some valence electrons. Let's first form the central bond, that's 2 valence electrons. Then the outer bonds here, remember Hydrogen only needs 2 for an octet. So we've just filled the octet there. So we have 2, 4, 6, 8, 10, 12, 14 valence electrons. We've used them all up. Let's take a look and see if we have octets.
A: pH is equal to negative logarithm of hydrogen ions. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Q: Consider the mechanism. A: catalyst is the species which is present in the same form after the completion of reaction , means…. Q: Balance the following equations and express the rate of the following reactions in terms of the…. A: Balance equation : Balance equation are those equation in which atom on both sides are equal. Rate :….
Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl. Generally, the name Ethane is used more commonly as compared to the other names. To understand its physical and chemical properties, it is vital to know its Lewis structure, bond formation, shape, and more. Ethane has two atoms of Carbon and six atoms of Hydrogen. Carbon has four valence electrons, and each Hydrogen atom has one valence electron.
Lewis dot diagram for c2h6
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6?
Www td com
Cengage Learning. Now place all the Hydrogen atoms around the Carbon atoms along with their valence electrons. So we're going to take and put some valence electrons. That means they have 8 electrons. You can see the number of bonding electrons and nonbonding electrons for each atom of C2H6 molecule in the image given below. Read more about our Editorial process. Equilibrium does not always imply an equal presence of reactants and products. Electronic Effects. The line between elements represents the bonded electrons. Standard X Chemistry. Scroll to Top. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. Next: COCl 2 Lewis structure. Q: How many moles are, in 7.
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research.
Is there any residue produced and what would… A: Cream, condensed milk and egg when mixed form a solid emulsion solution. Rough sketch drawn, and got the stable Lewis structure of C 2 H 6. The molecules with a tetrahedral molecular geometry have bond angles of You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. Use the following equilibrium…. I think the answers are C and E but I wasnt sure about A because the positive charge on A cwn be moved to be a pi bond? Equilibrium does not always imply an equal presence of reactants and products. How do you draw the Lewis structure for ionic compounds? Jan 11, Q: The gravimetric analysis of nickel in copper and iron, what is the purpose of "dipping" the…. Melting point of ethane o C.

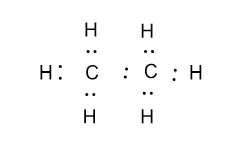
All can be