Lewis structure of sf2
Q: How would you prepare Q: For iron in a low spin state O a. A: We have to find the low spin state of iron.
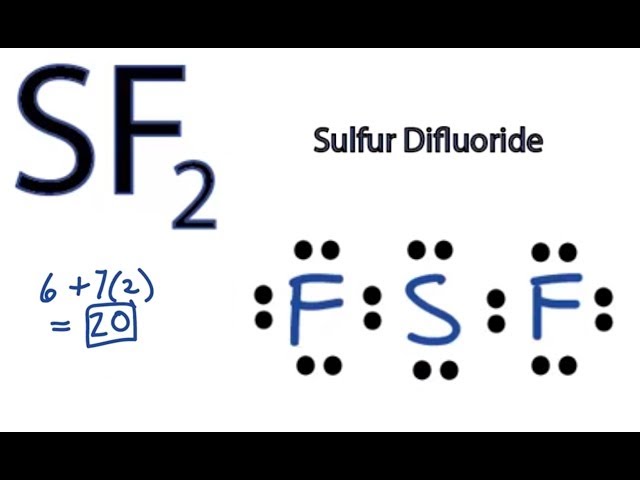
Wiki User. The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Ensure that Flourine is not breaking the octet rule as it is a smaller atom and is not known to do so. They should both have neutral charges so no charge is required to be written. So sulfur will end up with 6 electron in its valence level. This molecule doesn't follow the octet rule.
Lewis structure of sf2
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape. Lewis Structure of SF2, the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. The two lone pairs of electrons push the Fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent. Your physics assignments can be a real challenge, and the due date can be really close — feel free to use our assistance and get the desired result. Be sure that math assignments completed by our experts will be error-free and done according to your instructions specified in the submitted order form. Our experts will gladly share their knowledge and help you with programming projects. Organic Chemistry. Arrange the atomic cores for the two fluorine atoms in SF2 with the sulfur atom as the main atom. Rewrite the atomic cores placing an electron pair between each flourine and sulfur core Write the Lewis structure of SF2 in the box,distributing the remaining valence electrons so that all three atoms are in accordance with the octet rule. Sulfur difluoride is a covalent compound.
The Electron Configuration. The Alkyl Groups. Our experts will gladly share their knowledge and help you with programming projects.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
The Sulfur atom S is at the center and it is surrounded by 2 Fluorine atoms F. The Sulfur atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SF2. Here, the given molecule is SF2 sulfur difluoride.
Lewis structure of sf2
SF2 consists of one sulfur S atom and two fluorine F atoms. Sulfur is in Group 16 of the periodic table and has six valence electrons, while fluorine is in Group 17 and has seven valence electrons. Calculate the Total Number of Valence Electrons.
Jimmy choo high heel boots
O nonpolar. Density of Geometric Objects. Chapter Questions Section: Chapter Questions. Lewis Dot Symbols. Use standard thermodynamic data…. No, SF6 doesn't. SF2 can be represented by the Lewis dot structure of Solutions: Solubility and Intermolecular Forces. Boron Family Reactions. Titrations: Weak Acid-Strong Base. Face Centered Cubic Unit Cell. Band of Stability: Beta Decay. Chemical Properties. Table of contents.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride.
A: Given are haworth projections of carbohydrate molecules. Expert Solution. Does CaCO3 have a Lewis structure? Benzene Reactions. Include stereochemistry when… A: The reaction is initiated by the formation of free radicals. Significant Figures: Precision in Measurements. What is the Lewis dot structure for SF2? Combustion Apparatus. A: Given is organic reaction. Arrhenius Acids and Bases. Titrations: Weak Acid-Strong Base. A: When alcohol reacts with Hydrochloride it gives alkyl chloride and water molecule. Q: Curved arrows are used to illustrate the flow of electrons. There will be four bonds to each carbon atom.

Prompt reply)))