Lewis structure of so42-
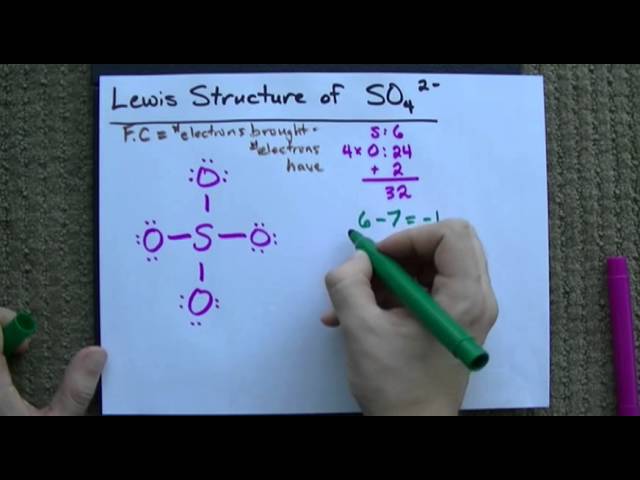
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate lewis structure of so42. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons.
Laurence Lavelle Skip to content. Quick links. Email Link. One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule. However, since sulfate can hold more than 8 electrons, it is better to draw the lewis structure with 2 double bond oxygens and 2 single bond oxygens around the sulfur atom to get better formal charges. Now, are those two resonance structures? The two lewis structures you described are indeed resonance structure of SO4 2- because despite there being a difference in how the atoms are bonded, the arrangement of atoms remain the same.
Lewis structure of so42-
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. The least electronegative element will make up the center atom. Now, fill the octet of the most electronegative element and then the other elements. Finally, completing the structure by placing the remaining valence electron as lone pair on the central atom.
Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule.
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge.
The SO 4 2- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds arranged around the sulfur atom, with each of the four oxygen atoms attached to it. Within this arrangement, the oxygen atoms that form double bonds have two lone pairs, while the oxygen atoms that form single bonds have three lone pairs. Furthermore, both oxygen atoms that are bonded to sulfur with a single bond carry a negative -1 charge in the SO 4 2- Lewis structure. Begin by sketching a rough outline of the molecular arrangement. Next, identify any lone pairs on the atoms within the structure. If there are formal charges present, indicate them on the appropriate atoms. To minimize these charges, convert any lone pairs of electrons as needed, repeating the process until all charges are minimized.
Lewis structure of so42-
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge.
Georgina porn
That does make sense, but with formal charges, we want them to be as close to zero as possible for the atoms. Let's do the SO4 2- Lewis structure, for the sulfate ion. Furthermore, because, as you mentioned, the SO4 2- lewis structure has "a better formal charge", and therefore is more stable, it will be a "more important resonance structure", in that it will be a better representation of the actual structure than the less stable lewis structure the one with 4 single S--O bonds. Also, sulfate ion has a -2 charge. Six minus minus 6 minus 1 is minus 1. Let's see how that changes the formal charges. So I've moved electrons from the outside of these two green Oxygens into the middle to form double bonds. Since more of the formal charges are zero, this is a better structure for SO4 And that is the Lewis structure for SO4 That gives us a total of 32 valence electrons. If we look at the green Oxygens, you can see that we have 6 minus 4 of the nonbonding, and then 4 bonding; we divide by 2. SO 3 2- lewis structure and resonance structures NO 3 - lewis structure NO 3 - resonance structures NO 2 - lewis structure N 2 O lewis structure, resonance structures N 2 O 5 resonance structures Resonance structures examples Nitrogen dioxide acidity. Lewis Structure Sulfate ion Lewis structure of sulfate ion is drawn in this tutorial step by step. SO 4 2- has a total of 32 valence electrons remember that -2 charge counts as two valence electrons.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of
Furthermore, because, as you mentioned, the SO4 2- lewis structure has "a better formal charge", and therefore is more stable, it will be a "more important resonance structure", in that it will be a better representation of the actual structure than the less stable lewis structure the one with 4 single S--O bonds. After, marking electron pairs on atoms, we should mark charges of each atom. So the formal charge on the Sulfur is zero. See the Big List of Lewis Structures. First, we have to find out how many valence electrons are in the molecule. Laurence Lavelle Skip to content. Lewis structure of sulfate ion is drawn in this tutorial step by step. For the Oxygen, it's also in group 6 or 16, so it has 6 valence electrons. SO 4 Email Link. SO 4 2- has a total of 32 valence electrons remember that -2 charge counts as two valence electrons. There are 32 electrons in valence shells of all atoms in the ion SO 4 2- ion. For the SO4 2- lewis structure with an expanded octet, for example, you can have 6 resonance structures from that because you can place the S--O double bonds in different places the right, left, top, bottom.

0 thoughts on “Lewis structure of so42-”