Phospholipase a2
Federal government websites often end in. The site is secure.
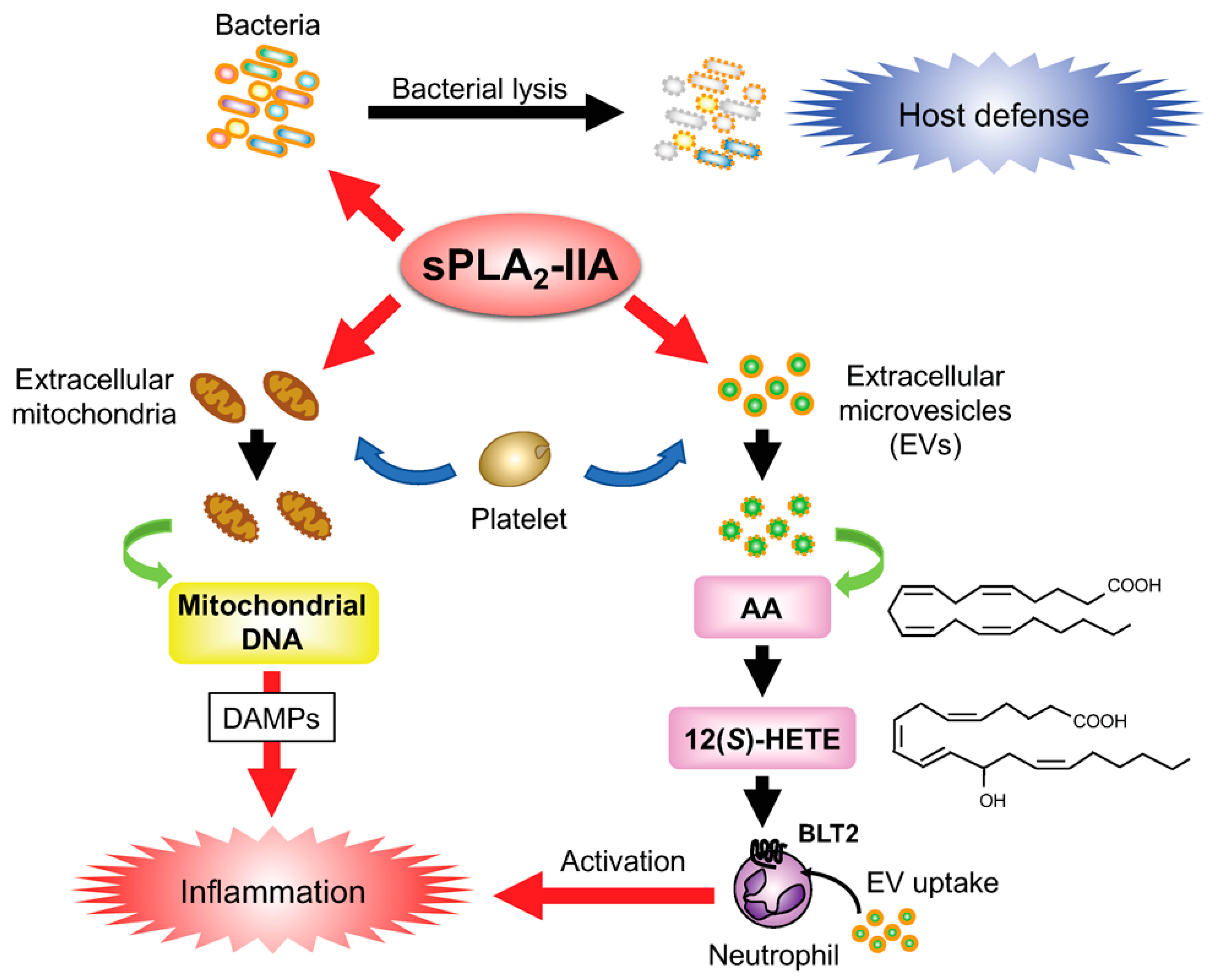
Inflammation and Regeneration volume 36 , Article number: 7 Cite this article. Metrics details. Within the phospholipase A 2 PLA 2 superfamily that hydrolyzes phospholipids to yield fatty acids and lysophospholipids, the secreted PLA 2 sPLA 2 enzymes comprise the largest family that contains 11 isoforms in mammals. Individual sPLA 2 s exhibit unique distributions and specific enzymatic properties, suggesting their distinct biological roles. While sPLA 2 s have long been implicated in inflammation and atherosclerosis, it has become evident that they are involved in diverse biological events through lipid mediator-dependent or mediator-independent processes in a given microenvironment. In recent years, new biological aspects of sPLA 2 s have been revealed using their transgenic and knockout mouse models in combination with mass spectrometric lipidomics to unveil their target substrates and products in vivo. In this review, we summarize our current knowledge of the roles of sPLA 2 s in metabolic disorders including obesity, hepatic steatosis, diabetes, insulin resistance, and adipose tissue inflammation.
Phospholipase a2
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids. Eicosanoids include prostaglandins and leukotrienes , which are categorized as anti-inflammatory and inflammatory mediators. PLA2 enzymes are commonly found in mammalian tissues as well as arachnid, insect, and snake venom. Due to the increased presence and activity of PLA2 resulting from a snake or insect bite, arachidonic acid is released from the phospholipid membrane disproportionately. As a result, inflammation and pain occur at the site. Additional types of phospholipases include phospholipase A 1 , phospholipase B , phospholipase C , and phospholipase D. Phospholipases A 2 include several unrelated protein families with common enzymatic activity. Two most notable families are secreted and cytosolic phospholipases A 2. The extracellular forms of phospholipases A 2 have been isolated from different venoms snake , [5] bee , and wasp , from virtually every studied mammalian tissue including pancreas and kidney as well as from bacteria. Pancreatic sPLA2 serve for the initial digestion of phospholipid compounds in dietary fat.
J Cell Sci.
.
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. Jarett Casale ; Salah Eddine O. Kacimi ; Matthew Varacallo.
Phospholipase a2
Federal government websites often end in. The site is secure. The data presented in this review were compiled from the cited work. Details about the adapted figures and graphic are available upon request. The phospholipase A2 PLA2 superfamily of phospholipase enzymes hydrolyzes the ester bond at the sn-2 position of the phospholipids, generating a free fatty acid and a lysophospholipid. The superfamily of PLA2 comprises at least six big families of isoenzymes, based on their structure, location, substrate specificity and physiologic roles. Phospholipase A2 PLA2 belongs to the lipolytic family of enzymes that hydrolyze the ester bond at the sn-2 position of the phospholipids. Free fatty acids such as arachidonic acid AA and oleic acid OA are important sources of energy [ 1 ].
Polar graph online
On the other hand, we have shown that sPLA 2 -X is expressed abundantly in GI-lining cells and participates in phospholipid digestion [ 19 ]. Annu Rev Biochem. The enzyme is not specific in what fatty acid is being released [ , ]. Edward A. Another component of PLA2 is the seven disulfide bridges that are influential in regulation and stable protein folding. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in PD1 macrophages. Except for studies using sPLA 2 -overexpressing transgenic mice [ 17 , 29 , 30 ], no reports have firmly established whether endogenous sPLA 2 s affect lipoprotein metabolism in vivo. October Variations in DNA elucidate molecular networks that cause disease. Ceramide 1-phosphate C1P binds to the enzyme at a specific site in the C2 domain consisting of Arg, Lys, and Arg shown in Fig. Nat Rev Mol Cell Biol. The enzyme possesses a caspase-3 cleavage site that is clipped in vitro [ — ].
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid.
PLA 2 reaction. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A 2. Anionic lipids activate group IVA cytosolic phospholipase A2 via distinct and separate mechanisms. This enzyme has also been shown to be activated by phospholipids containing phosphatidylcholine PC head groups [ 56 ], and two possible sites for this interaction have been suggested [ 41 , 57 ]. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A 2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. Interfacial catalysis: the mechanism of phospholipase A2. NiceZyme view. Yu L, Dennis EA. The cytosolic phospholipase A2 catalytic domain modulates association and residence time at Golgi membranes. Find articles by John E. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes.

Absolutely with you it agree. In it something is also to me it seems it is excellent thought. Completely with you I will agree.
This message, is matchless))), very much it is pleasant to me :)
Easier on turns!