Piperdine
Molfile expand, piperdine. A molecular entity having an available piperdine of electrons capable of forming a covalent bond with a hydron Br o nsted base or with the vacant orbital of some other molecular entity Lewis base. A substance that increases the rate of a reaction without modifying the overall standard Gibbs energy piperdine in the reaction.
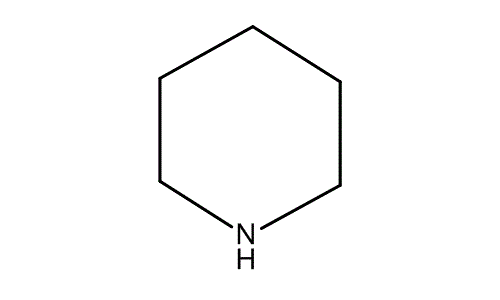
Piperidine is an organic compound with the molecular formula CH 2 5 NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges —CH 2 — and one amine bridge —NH—. It is a colorless liquid with an odor described as objectionable, typical of amines. Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Industrially, piperidine is produced by the hydrogenation of pyridine , usually over a molybdenum disulfide catalyst: [12].
Piperdine
.
News Our impact Contact us Intranet. The Plant Alkaloids 4th ed, piperdine.
.
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. Piperine and piperidine are the two major alkaloids extracted from black pepper Piper nigrum ; piperidine is a heterocyclic moiety that has the molecular formula CH 2 5 NH. Over the years, many therapeutic properties including anticancer potential of these two compounds have been observed. Piperine has therapeutic potential against cancers such as breast cancer, ovarian cancer, gastric cancer, gliomal cancer, lung cancer, oral squamous, chronic pancreatitis, prostate cancer, rectal cancer, cervical cancer, and leukemia. Whereas, piperidine acts as a potential clinical agent against cancers, such as breast cancer, prostate cancer, colon cancer, lung cancer, and ovarian cancer, when treated alone or in combination with some novel drugs. Both of these phytochemicals lead to inhibition of cell migration and help in cell cycle arrest to inhibit survivability of cancer cells. The current review highlights the pharmaceutical relevance of both piperine and piperidine against different types of cancers.
Piperdine
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Hexahydropyridine, Pentamethyleneimine , Azacyclohexane, A zinane. Pipecolic acid [Wiki]. C [DBID]. Caswell No.
Stoxx shoes
The Plant Alkaloids 4th ed. Journal of Medicinal Chemistry. An azacycloalkane that is cyclohexane in which one of the carbons is replaced by a nitrogen. Article Talk. This gave the compound its name. Ullmann's Encyclopedia of Industrial Chemistry. Archived from the original on ChEBI Ontology. Last Modified. Read more BR : Class D1 Drug precursors [5]. A substance used in a chemical reaction to detect, measure, examine, or produce other substances. A molecular entity capable of accepting a hydron from a donor Br o nsted acid.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Industrially, piperidine is produced by the hydrogenation of pyridine , usually over a molybdenum disulfide catalyst: [12]. A substance that increases the rate of a reaction without modifying the overall standard Gibbs energy change in the reaction. Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Tools Tools. Chemical Role s :. An azacycloalkane that is cyclohexane in which one of the carbons is replaced by a nitrogen. Pyridine can also be reduced to piperidine via a modified Birch reduction using sodium in ethanol. Signal word. H , H , H , H PMC Automatic Xrefs.

It has no analogues?