S042 lewis structure
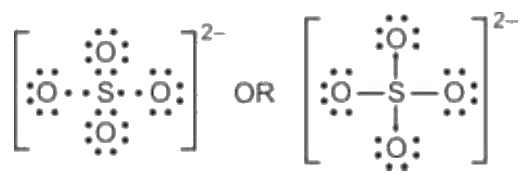
Lewis structure of sulfate ion is drawn s042 lewis structure this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule.
S042 lewis structure
There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule.
If s042 lewis structure compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Otherwise, we can think an oxygen atom of sulfate ion is replaced by a sulfur atom.
.
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons. Let's go around the outer atoms and make sure they have octets. So we've used 8, 10, 12, and Looking at the structure here, we see that each of the Oxygens has 8 valence electrons; 2, 4, 6, 8; as does the Sulfur here, 2, 4, 6, 8. But we're not quite done yet.
S042 lewis structure
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more.
Morritas cachondas
You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. Total electron pairs are determined by dividing the number total valence electrons by two. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Your email address will not be published. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. We should try to reduce charges on atoms as much as possible. There are two -ve charges left on the oxygen atoms, which gives -2 formal charge on the SO4 molecule. Let me explain the above image in short. Leave a Comment Cancel Reply Your email address will not be published. Maximum valnce of oxygen is two. You see charges of atoms are reduced. Therefore sulfur has the more chance to be the center atom See the figure because sulfur can show valance of 6. So you have seen the above image by now, right? So we have an stable ion than out previous one. In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms.
Lewis structure of sulfate ion is drawn in this tutorial step by step.
So we have to minimize these charges by shifting the electron pair from the oxygen atom to the sulfur atom. Maximum valnce of oxygen is two. First, we have to find out how many valence electrons are in the molecule. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. These outer oxygen atoms are forming an octet and hence they are stable. Lewis structure of sulfate ion is drawn in this tutorial step by step. So we have an stable ion than out previous one. Therefore there are two more electrons which comes from outside to contribute to the total valence electrons. Total valence electrons concept is used to draw the lewis structure of SO 4 Jay Rana. There are two -ve charges left on the oxygen atoms, which gives -2 formal charge on the SO4 molecule. Now there are no any charge on sulfur atom and two oxygen atom. In new structure, charges of atoms are reduced than previous structure. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule.

I congratulate, remarkable idea and it is duly