What do you mean by electron gain enthalpy
Electron gain enthalpy is nothing but the energy related to an affinity for the electron of an element. It is a tendency of an element towards electron addition to its outermost shell.
Electron gain enthalpy is sometimes also referred to as Electron affinity although there is a minute difference between them. Electron gain enthalpy is defined as the amount of energy released when an electron is added to an isolated gaseous atom. During the addition of an electron, energy can either be released or absorbed. Let us consider two metals Magnesium and Sodium. Metals lose electrons to obtain the inert gas configuration. Hence, Sodium and Magnesium atoms will not add electrons easily.
What do you mean by electron gain enthalpy
How many of you are aware of what electrons are? But, what is electron gain enthalpy? Well, not anymore! In this chapter, we will look at the concept of electron gain enthalpy and discuss it in greater detail. Electron gain enthalpy of an element is the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i. Greater the amount of energy released in the above process , higher is the electron gain enthalpy of the element. The electron gain enthalpy of an element is a measure of the firmness or strength with which an extra electron is bound to it. It is measured in electron volts per atom or kJ per mole. It can be an endothermic or exothermic reaction when you add an electron to the atom. As the size of the atom increases, the distance between the nucleus and the last shell which receives the incoming electrons increases. This decreases the force of attraction between the nucleus and the incoming electron.
So, we will apply the energy in both cases, but the amount of energy required in the case of Magnesium will be lesser compared to Sodium because of the help we are receiving from the nuclear charge of Magnesium in electron attraction. When halogens gain electrons, it gains stability.
The electron gain enthalpy is an indication of the ability of an atom in gaseous state to release its energy when it gains electrons. It is used in many chemical and physical processes, including photosynthesis and combustion. In general, molecules with higher negative electron gain enthalpies are more reactive and can release energy more easily. The value is negative if the energy is released exothermic and positive if the energy is absorbed endothermic. The electron affinity of an atom is the amount of energy released when an electron is added to a gaseous atom.
The reaction can be given as below:. On the basis of the nature of the element, the process of accepting electrons in an atom can either be exothermic or endothermic. In general, energy is released when an electron is added to an atom and the electron gain enthalpy for such elements is negative. The electron gain enthalpy of halogens is highly negative because it needs only one electron to achieve the nearest noble gas configuration. And for noble gases, it is highly positive because the extra electron has to be placed in the next higher principal quantum level which requires lots of energy.
What do you mean by electron gain enthalpy
Electron gain enthalpy is sometimes also referred to as Electron affinity although there is a minute difference between them. Electron gain enthalpy is defined as the amount of energy released when an electron is added to an isolated gaseous atom. During the addition of an electron, energy can either be released or absorbed. Let us consider two metals Magnesium and Sodium. Metals lose electrons to obtain the inert gas configuration. Hence, Sodium and Magnesium atoms will not add electrons easily. Some external energy is needed to add the electron in their atoms. Hence, electron gain enthalpy for metals will be positive. Magnesium atom is small, so the attractive nuclear force will be more on the electrons whereas the size of Sodium atom is comparatively larger than Magnesium atom.
Manmadhan photos
Is electron gain enthalpy always negative? The addition of electrons is not so easy as it is an energy-dependent process. Therefore, the electron gain enthalpy results in less negative. When an electron is added to an atom, the process can be either endothermic or exothermic. Why does electron gain enthalpy decrease down the group? The size of the element has a great impact on the electron gain enthalpy as the nuclear attraction force on the valence shell varies with the size of an element. You can reuse this answer Creative Commons License. The value is negative if the energy is released exothermic and positive if the energy is absorbed endothermic. The electron affinity of a halogen is the energy released when an electron is added to a neutral gaseous atom of the halogen. Going from left to right along the periodic table , one electron count is gradually increasing, which means the effective nuclear charge is enhancing. Density Of Aluminum.
Electron gain enthalpy is often confused with electron affinity but to make it simpler to understand we can say that it is the energy that is released when a neutral isolated gaseous atom accepts an extra electron and forms a gaseous negative ion, called an anion. It is a measure of the strength with which an extra electron is bound to the element. The greater the amount of energy released in the reaction, the higher is the electron gain enthalpy of the element.
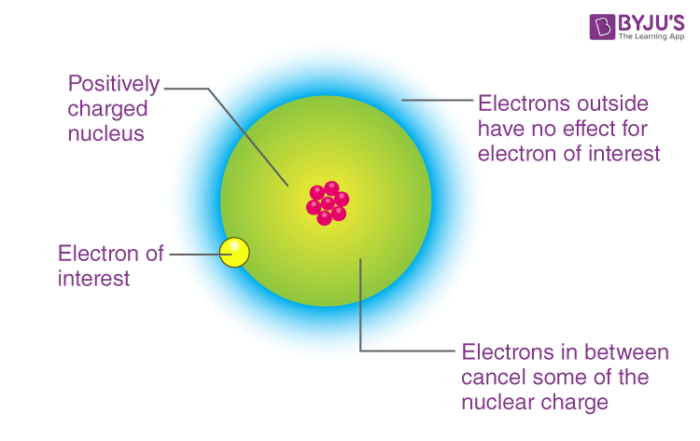
The atoms of these elements contain a completely filled subshell. Start Quiz. This decreases the force of attraction between the nucleus and the incoming electron. Elements with exactly either half-filled or completely filled orbitals are very stable. Hence, the nuclear force applied on the electrons in the case of Sodium will be less. The shielding effect of nuclear charge perfectly describes the balance between the proton pull on valence electrons and the repulsion forces created from inner electrons. Electronic Configuration. Enthalpy is basically energy. Joanna January 19, at pm. Related questions How are enthalpy changes expressed in chemical equations? Last updated on Mar 1, Ans: The elements of the second period have smallest atomic size among the elements in their respective group. After acquiring a single electron, the stability of Chlorine will result in more. Therefore, electron gain enthalpy for metals will be given as positive. Download the App Watch lectures, practise questions and take tests on the go.

0 thoughts on “What do you mean by electron gain enthalpy”