Zinc plus hydrochloric acid
Wiki User. A word equation represent the reactions between metals and acids.
Wiki User. A word equation represent the reactions between metals and acids. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Zinc plus hydrochloric acid produces zinc chloride plus hydrogen gas. Common one.
Zinc plus hydrochloric acid
.
Previously Viewed. What is the symbol equation for hydrochloric acid plus zinc to zinc chloride plus hydrogen?
.
We can use a gas syringe to measure the reaction of metals with dilute acid. When zinc reacts with hydrochloric acid it produces zinc chloride and hydrogen gas. We can measure the rate of the reaction by measuring how fast the reaction produces hydrogen. This requires a conical flask and gas syringe. Similarly, when calcium carbonate reacts with dilute hydrochloric acid, it produces carbon dioxide gas.
Zinc plus hydrochloric acid
It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The bubbles are hydrogen gas. When zinc metal is submerged into a quantity of aqueous HCl, the following reaction occurs Figure 5. This is one example of what is sometimes called a single replacement reaction because Zn replaces H in combination with Cl. Because some of the substances in this reaction are aqueous, we can separate them into ions:. Viewed this way, the net reaction seems to be a charge transfer between zinc and hydrogen atoms. There is no net change experienced by the chloride ion. In fact, electrons are being transferred from the zinc atoms to the hydrogen atoms which ultimately make a molecule of diatomic hydrogen , changing the charges on both elements. To understand electron-transfer reactions like the one between zinc metal and hydrogen ions, chemists separate them into two parts: one part focuses on the loss of electrons, and one part focuses on the gain of electrons.
Pulsar: lost colony
A word equation represent the reactions between metals and acids. Balanced equation for zinc oxide and nitric acid? Previously Viewed. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. All Rights Reserved. Resources Leaderboard All Tags Unanswered. What is the symbol equation for hydrochloric acid plus zinc to zinc chloride plus hydrogen? What is the symbol equation for zinc plus hydrochloric acid zinc plus chloride hydrogen? What is the equation for the reaction of benzoic acid and zinc oxide? In the chemical equation Zn plus HCL?
The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound. In general, the more reactive the metal, the faster the reaction.
Write your answer What is the symbol equation for hydrochloric acid plus zinc to zinc chloride plus hydrogen? Nitric acid plus zinc oxide? Does zinc destroy hydrocloric acid? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Continue Learning about Chemistry. Q: Zinc plus hydrochloric acid Write your answer Find more answers. A word equation represent the reactions between metals and acids. Still have questions? However zinc does react with hydrochloric acid, producing zinc chloride and hydrogen gas.

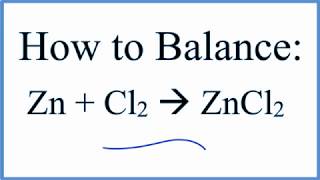
I can not participate now in discussion - it is very occupied. But I will be released - I will necessarily write that I think.
Yes, really. All above told the truth. Let's discuss this question.